A deep look into the Stern Layer:
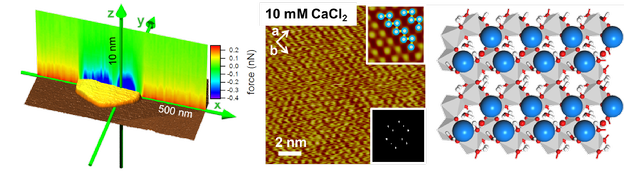
The distribution of ions and charge at solid-water interfaces plays an essential role in a wide range of processes in biology, geology and technology. While theoretical models of the solid-electrolyte interface date back to the early 20th century, a detailed picture of the structure of the electric double layer has remained elusive, largely because of experimental techniques have not allowed direct observation of the behaviour of ions, i.e. with subnanometer resolution. Making use of recent advances in high-resolution Atomic Force Microscopy, with atomic level precision, the ordered adsorption of the mono- and divalent ions that are common in natural environments to heterogeneous gibbsite/silica surfaces in contact with aqueous electrolytes was revealed. Complemented by density functional theory, the experiments produced a detailed picture of the formation of surface phases by templated adsorption of cations, anions and water, stabilized by hydrogen bonding.
This research was published in Scientific Reports: http://www.nature.com/srep/2014/140514/srep04956/full/srep04956.html
More recent news
 Thu 11 Dec 2025Three new NAE Fellows and Young Engineer with UT background
Thu 11 Dec 2025Three new NAE Fellows and Young Engineer with UT background Thu 11 Dec 2025Two UT projects receive Perspectief grant
Thu 11 Dec 2025Two UT projects receive Perspectief grant Fri 5 Dec 2025Transforming urban policy for a healthier and safer Enschede (and other cities)
Fri 5 Dec 2025Transforming urban policy for a healthier and safer Enschede (and other cities) Fri 28 Nov 2025UT celebrates 64th Dies: a look at the hospital of the future
Fri 28 Nov 2025UT celebrates 64th Dies: a look at the hospital of the future Fri 28 Nov 2025Van Damme scholarship for Lisa Deijlen and Wietske Woliner
Fri 28 Nov 2025Van Damme scholarship for Lisa Deijlen and Wietske Woliner
