A protein by the name of clathrin plays a key part in endocytosis, the process by which living cells absorb large molecules. The protein can form “cages”, in which these molecules become trapped. Until recently, the details of this process were not fully understood. Using computer simulations, researchers at the University of Twente have now uncovered the secrets of this mechanism. It seems that the traditional view of endocytosis was overly complex.
Cells use endocytosis to absorb large molecules. The cell membrane folds inwards, creating a pocket, which then pinches off to form a vesicle containing the target molecule. This vesicle is then transported to the site inside the cell where it is needed.
Old-fashioned football
Clathrin has long been known to play a pivotal role in the process of wrapping up these trapped molecules. The protein's part in this process is to form "cages" around a piece of cell membrane containing the target molecule. These cages are similar in structure to old-fashioned footballs, consisting only of pentagons and hexagons. Clathrin complexes were already known to form flat, honeycomb-like structures in cells, consisting only of hexagons. Until recently, however, it was not known how clathrin complexes switch from one structure to the other. As a result, the practical aspects of the process of endocytosis were also poorly understood. The most important question in this regard is how pentagons are suddenly introduced into a structure consisting of only hexagons. Scientists have been researching this matter for forty years.
Much simpler
The previous hypotheses were all based on complex mechanisms involving numerous intermediate steps. Using computer simulations, researchers at the University of Twente have now shown that the mechanism is much simpler than people had previously assumed. The study revealed that, when the flat structure starts to bend, tensions within the grid can cause a small highly-curved piece to break off. Successive individual clathrin molecules then bind to the curved piece until the protein cage is complete. The findings are consistent with previous studies in which detached, curved structures had been observed in special electron microscope images. However, previous interpretations of these images were incorrect.
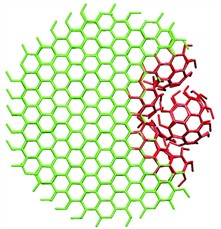
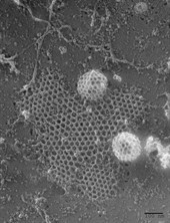
Image credits: John Heuser
Study
The study was conducted by Dr Wouter den Otter and Prof. Wim Briels of the Computational BioPhysics group at the University of Twente. After entering details of clathrin's properties into software of their own design, they can simulate the processes involved in the formation of protein cages. Their results will soon be published in Traffic, a leading scientific journal in the field of molecular transport by membranes. As statistical physicists, the researchers take great pride in the fact that they are able to publish their work in a biological journal. "It shows that our work is relevant to them".
Note to the press
For further details, or a digital version of the article entitled "The Generation of Curved Clathrin Coats from Flat Plaques", please contact Joost Bruysters, the Science Information Officer (+31-(0)53-4892773 / 0610488228).





