Researchers from the Faculty of S&T at the University of Twente have shown a novel approach for the design of efficient solar fuel devices. Research performed by graduated PhD student Kaijian Zhu shows that reducing the light-induced twisting of molecules can turn hydrogen generation on.
Photoelectrochemical cells are promising for the production of solar fuels, for example, the conversion of water into hydrogen or CO2 into organic molecules. However, their efficiency has been limited by the performance of the photocathode, one of the functional electrodes in these cells. Present research focused on manipulating the behaviour of molecules on the Nickel Oxide (NiO) surfaces of these photocathodes.
Twisting molecules
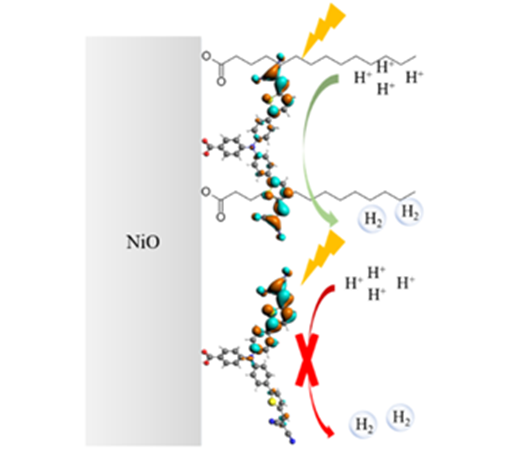
When light is absorbed by the dye molecules on the NiO surface, they twist to promote the separation of positive and negative charges. A key question was how this twisting affects the performance of the photocathode. Zhu’s research demonstrated that by reducing this twisting process, hydrogen generation under illumination can be turned on.
By adding a long chain, hydrophobic hydrocarbon (Myristic Acid) to the NiO surface next to the light-sensitive dye molecules, the researchers were able to control and reduce the degree of twisting of the dye molecules when light is absorbed. Very interestingly, the presence of Myristic Acid enabled light-induced hydrogen evolution in water even without a hydrogen evolution catalyst. “The hydrogen generation is likely a synergetic effect of inhibited twisting of the dye radical anion, increasing its electrochemical potential, combined with charge transfer and reduction of protons at the hydroxylated NiO surface”, says Annemarie Huijser, the corresponding author of this work.
The research illustrates the importance of understanding effects of light-induced intramolecular twisting and demonstrates that control thereof enables a straightforward design approach for efficient photocatalysis.
About the research
The research has been carried out by Dr. Kaijian Zhu, former PhD student in the Photocatalytic Synthesis Group at the University of Twente, supervised by Dr. Annemarie Huijser and Prof. Guido Mul. The project is part of the Advanced Research Center Chemical Building Blocks Consortium (ARC CBBC; www.arc-cbbc.nl).
Within the ARC CBBC, the University of Twente, the University of Amsterdam, and industrial partner Shell, among others, collaborated in this project within the central theme ‘Energy Transition’, while theoretical modelling was performed at the University of Leiden. Dr. Annemarie Huijser is Associate Professor in the Photocatalytic Synthesis group of the Faculty of S&T and the MESA+ Institute. Her research is focused on the control of light-induced processes in materials with application in solar energy conversion, by tuning the static or dynamic nanostructure. Complementary ultrafast spectroscopic methods are used to visualize light-induced processes in real time.
The article “Limiting Molecular Twisting: Upgrading a Donor–Acceptor Dye to Drive H2 Evolution”, by Dr. Kaijian Zhu (Faculty of S&T), Dr. Ainoa Paradelo Rodríguez (Faculty of S&T), Dr. Maria B. Brands (University of Amsterdam), Titus de Haas (Leiden University), Dr. Francesco Buda (Leiden University) , Prof. Dr. Joost N.H. Reek (University of Amsterdam), Prof. Dr. Guido Mul (Faculty of S&T) and Dr. Annemarie Huijser (Faculty of S&T) is recently published in Advanced Science and can be read online.





