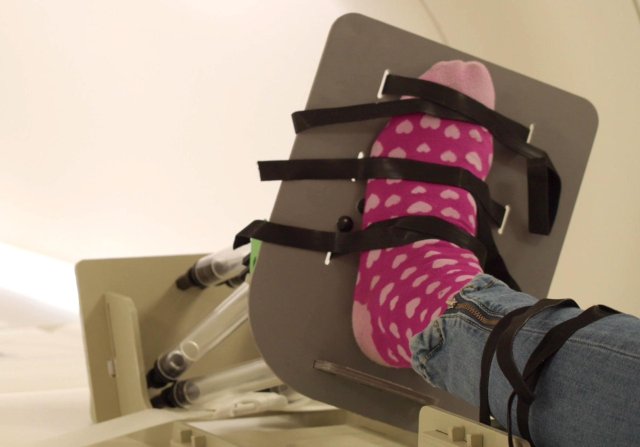
The University of Twente is introducing its first MDR-compliant open-source medical device: the 3d base plate. This open-source initiative provides an alternative route to low-risk medical devices that are not commercially viable. However, it does have a clinical added value for the patient. One such non-commercially viable tool is the 3d footplate, a rearfoot pathology medical imaging tool. Soon to be made by doctors at a cost price with corresponding MDR documentation via open-source.
Within the chair, Biomedical Device Design & Production UT (TechMed Center) is investigating alternative routes to make medical devices available as an open-source medical device, including related MDR documentation (Medical Device Regulation). This enables medical professionals to produce the devices at a cost price and reduce the burden of documentation. The 3d base plate is the first open-source UT's medical device, but certainly not the last.
A difficult profitable revenue model
The development and market introduction of medical devices often requirea lot of time and cost. In addition to compliance with strict MDR regulations, financing and drawing up a business case are also major challenges. Especially for devices aimed at small patient groups or rare diseases, it is difficult to set up a profitable revenue model. As a result, many innovative tools never reach the users who could benefit from them.
The 3D footplate
An example of such a non-commercially viable tool is the 3d footplate, a medical imaging tool for complex hindfoot pathologies. This 'Class I Medical Device' helps to position the foot to the lower leg, allowing clinical stress tests to be simulated during CT scans. The images obtained provide quantitative data, such as relative bone movements that contribute to the diagnosis and assessment of surgical procedures. Since this tool is mainly used in academic medical centres, such as the AUMC and the MUMC, and the potential market is small, a traditional business model is not feasible.
IKEA style
In order to make this tool accessible to the clinicians concerned, the UT has redesigned the original 3d base plate according to the 'Design for Assembly methodology'. This resulted in an IKEA-style prototype that is easy to manufacture. With laser-cut parts, 3d printing technology and standard components. This keeps production costs low, and assembly is simple.
Producing at cost price
In addition, most of the MDR documentation has been prepared using UT templates. Including a risk analysis, risk mitigation strategies, evaluation tests, an assembly manual and the technical file. This complete package – including bill of materials, technical drawings and documentation – is offered via an open-source platform. Medical centres can thus produce the base plate at cost price and adapt the documentation to their quality management system.
Global impact and improvement of healthcare
The 3d base plate is the UT's first open-source medical device, but certainly not the last. The Biomedical Device Design & Production chair continues to work on new cases and a broader design strategy for 'Class I Medical Devices'. Offering these tools open-source not only increases accessibility in the Netherlands, but also creates the opportunity for cooperation in low- and middle-income countries. This contributes to the global impact of this initiative and the improvement of healthcare.
About the University of Twente
The University of Twente is a leading technical university in the Netherlands, focused on innovation and interdisciplinary research. The Biomedical Device Design & Production chair focuses on the development of medical technologies that are accessible and practically applicable for clinical practice.
More recent news
 Wed 4 Mar 2026Twente Health School Event: ready to shape the future of health?
Wed 4 Mar 2026Twente Health School Event: ready to shape the future of health? Wed 11 Feb 2026Herman van der Kooij awarded NWO grant for E-Walk project
Wed 11 Feb 2026Herman van der Kooij awarded NWO grant for E-Walk project Thu 5 Feb 2026Innovative toys turn hand therapy into a more enjoyable and effective experience for children with cerebral palsy
Thu 5 Feb 2026Innovative toys turn hand therapy into a more enjoyable and effective experience for children with cerebral palsy Wed 21 Jan 2026STAR interview: reshaping tomorrow through additive manufacturing of energy materials
Wed 21 Jan 2026STAR interview: reshaping tomorrow through additive manufacturing of energy materials Tue 20 Jan 2026Pioneers in Healthcare Award ceremony 2025
Tue 20 Jan 2026Pioneers in Healthcare Award ceremony 2025