Ph.D. students: Josée Kleibeuker and Michelle Kruize
Daily supervisor: Guus Rijnders
In many ionic materials, such as complex oxide materials, surfaces created along specific crystalline directions become electrically polarized. Equally, such electronic polarization can occur at the interface between two different materials.[1] This polarization implies that the electrostatic potential would diverge when moving from the interface into the bulk, as will be shown in more detail below. This divergence is energetically not allowed, leading to a ‘polar catastrophe’. Reconstructing the electronic configuration of the interface, for example by charge transfer across the interface or into the bulk layers, or by the creation of crystal defects such as oxygen vacancies is one of the ways to resolve this problem.
A good way to illustrate the polar catastrophe is by considering the perovskite-type oxides. These materials are commonly described in terms of their cubic unit cells, with the generic formula ABO3. To understand their interface behavior, it is however more instructive to look at these materials in terms of their constituting AO and BO2 layering sequence. For example, whereas the electrical insulators SrTiO3 and LaAlO3 are seemingly similar, the Sr2+O2- and Ti4+(O2-)2 layers are charge-neutral, while the charge states in the LaAlO3 are positive for La3+O2- and negative for Al3+(O2-)2. When stacking these materials, creating a heterostructure, the AO-BO2 sequence is maintained, and consequently a polarity discontinuity arises at the LaAlO3 - SrTiO3 interface. The resulting alternation of charged layers causes a build-up of the electric potential in the film, which for energetic reasons cannot be sustained (figure 1a). This ‘polar catastrophe’ therefore drives a reconstruction of the interface.
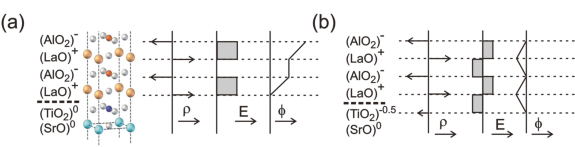
Figure 1. LaAlO3 is a polar material with charged (〉) layers that induce an electric field (E) within the material and a potential (⎞) build-up. (a) The interface between LaAlO3 and SrTiO3 gives rise to a diverging potential. (b) A transfer of half an electron per unit cell to the terminating TiO2 plane provides a non-diverging potential and a stable interface (after [2]).
One of the consequences of this reconstruction is the fact that these interfaces exhibit extraordinary properties. Conductive planes and magnetic properties have been found at the interface of otherwise two insulating perovskites [3,4]. Furthermore superconductivity and the existence of quantum Hall effect is shown in these interfaces, leading to a great deal of attention [5,6].
It will be of great importance to understand at which point during growth the essential electronic reconstruction processes take place. The kinetics, thermodynamics and electrostatics during the growth process each have their own energy scales, which should be compared to the energy associated with the potential build-up at a polar hetero-epitaxial interface. PLD allows one to manipulate and investigate these scales with great flexibility. In-situ Atomic Force Microscopy (AFM) and in-situ Reflective high energy electron diffraction (RHEED) can establish growth modes and help to understand the kinetics of the growth, which can be employed under growth conditions, i.e. at temperatures above 700 C and a varying range of oxygen pressures. In-situ X-ray photoelectron spectroscopy (XPS) and Ultraviolet photoelectron spectroscopy (UPS) can determine the valence state of the ions, thereby giving direct information on the electronic reconstruction that takes place.
An important question is how a polar surface influences the initial nucleation and subsequent growth. Studying this presents a major, yet extremely interesting, scientific challenge, and will also provide a guideline for thermodynamically stabilizing novel phases, for example by applying electrostatic fields during growth or cool down.
Another aspect is this research is to find novel material combinations. Up to now, electronically reconstructed interfaces haven only been obtained on SrTiO3 substrates with its charge-neutral sub unit-cell layers. When growth on other materials with polar surfaces is also understood and developed, this would open up great potential for different materials combinations. Every materials combination brings along different physical and chemical questions as how to realize single-terminated surfaces and how the growth settings depend on the constituent atoms and their chemical bonding characteristics.
The research will focus primarily on interfaces involving model system such as titanates, vanadates, cuprates and magnetic oxides.
.
[1] A. Ohtomo and H.Y. Hwang, Nature 427, 423 (2004).
[2] N. Nakagawa, H.Y Hwang, and D.A. Muller, Nature Materials 5, 204 (2006).
[3] A. Brinkman, M. Huijben, M. van Zalk, J. Huijben, U. Zeitler, J.C. Maan, W.G. van der
Wiel, G. Rijnders, D.H.A. Blank, and H. Hilgenkamp, Nature Materials 6, 493 (2007).
[4] M. van Zalk, J. Huijben, A.J.M. Giesbers, M. Huijben, U. Zeitler, J.C. Maan, W.G. van der
Wiel, G. Rijnders, D.H.A. Blank, H. Hilgenkamp, and A. Brinkman, cond-mat\0806.4450
(2008). [subm. to Phys. Rev. Lett.].
[5] N. Reyren, S. Thiel, A. D. Caviglia, L. Fitting Kourkoutis, G. Hammerl, C. Richter, C. W.
Schneider, T. Kopp, A.-S. Rüetschi, D. Jaccard, M. Gabay, D. A. Muller, J.-M. Triscone,
and J. Mannhart, Science 317, 1196 (2007).
[6] Tsukazaki, A. Ohtomo, T. Kita, Y. Ohno, H. Ohno and M. Kawasaki, Science 315, 1388
(2007).
