Title: Materials for photoconversion
Executive organisational unit: Rijnhuizen
Project leader: Dr. ir. J.M. Sturm (j.m.sturm@utwente.nl)
Duration: 2008 - 2011
Partner(s): TU Delft, dr. ir. Roel van de Krol
Objectives
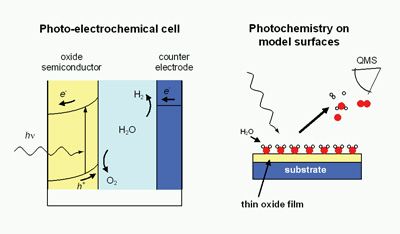
To develop model systems for and investigate the fundamental aspects of photo-electrochemical cells for production of hydrogen from sunlight and water.
Background, relevance and implementation
The use of fossil fuels as energy supply is regarded to be the major contribution to the climate change. Moreover, the sources of these fuels are depleting, such that alternative sources of energy are highly wanted. Our future energy supply will possibly be a mix of various renewable energy sources, of which solar energy is expected to be an important component. The sun delivers 3x1024 joules of energy per year, which is 10,000 times the current global energy consumption. However, harnessing a sufficient fraction of this energy is not straightforward. Solar energy from conventional solid state solar cells is currently too expensive compared to other renewable sources. Metal oxide semiconductors, like TiO2, are cheap and widely available, but make inefficient use of sunlight due to their large band gaps. A large breakthrough in the use of oxide semiconductors in photo-electrochemical cells was achieved by coating a mesoporous TiO2 film with a dye. The dye can be excited by visible light photons, followed by transfer of the generated electron to the TiO2 conduction band. Photo-electrochemical cells with aqueous electrolytes can be used for photo-induced splitting of water in hydrogen and oxygen, thereby providing a clean chemical fuel.