
PhD Student
E-mail: k.c.andree@utwente.nl
Telephone: +31 53 489 4187
Fax: +31 53 489 3511
Address: Faculty of Science and Technology
Medical Cell BioPhysics (MCBP)
Building: Carré
Room: CR4435
Drienerlolaan 5
P.O. Box 217
7500 AE Enschede
The Netherlands
Project goals
Project goals focus on technology development and evaluation to improve upon current state of the art techniques in detection and analysis of circulating tumor cells (CTC). The EU IMI project CANCER-ID aims to validate technologies for CTC, ctDNA and circulating microRNAs as blood-based biomarkers. Research will focus on technology for CTC enumeration, CTC isolation, CTC characterization and nucleic acid extraction based on clinical needs and utility. Within the EU FP7 CTCTrap project research will be conducted on the CTC isolation and identification from the CTCTrap device.
Circulating Tumor Cells a Real Time Liquid Biopsy
In cancer patients the major cause of death is due to metastatic disease rather than the solid primary tumor. In the process of cancer metastasis, tumor cells detach from their primary site, enter the circulatory system, migrate through the body and form secondary tumors at distant sites. These cells are known as circulating tumor cells. Peripheral blood represents a minimally invasive source of spreading tumor cells and could be used as a liquid biopsy for diagnosis and to monitor treatment and patient outcome. Before CTC can be established as a routine liquid biopsy in the clinic they will need to be present and isolated in sufficient numbers and their content readily accessible to determine whether certain therapies can be effective or not.
At present the only validated assay for CTC detection that has been cleared by the U.S. Food and Drug Administration is the CellSearch system. The number of cells that are detected with the CellSearch system in patients with metastatic carcinomas is in most cases too small to reliably determine heterogeneity and to be representative as a liquid biopsy. Approaches to increase blood volume are necessary to be able to detect more CTC. For CANCER-ID a diagnostic leukapheresis (DLA) will be used to obtain a product volume of 40 mL representing 750mL of blood, this will be collected and analyzed for the presence of CTC using different technologies. Within the CANCER-ID project alternative technologies are being developed to analyze 18mL DLA product. The remaining sample will be analyzed using the CellSearch system and the CTCTrap device.
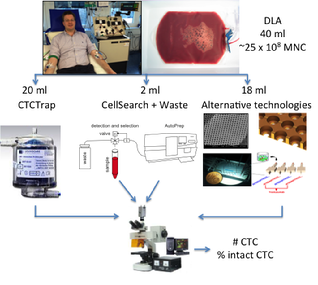
Figure 1: Tools used to isolate, detect and extract CTC from diagnostic leukapheresis products.
CD45 depletion
CTC are present at a ratio of about 1 CTC to 107 leukocytes. This huge number of hematopoietic cells in the background contributes to the difficulty in isolating CTC. Depletion of white blood cells is needed to be able to isolate CTC from a leukapheresis product. For this purpose we will test and compare multiple CD45 depletion techniques. Figure 2 shows the result of a blood sample spiked with tumor cells before and after depletion of CD45 positive cells.

Figure 2: FACS analysis of a leukapheresis sample spiked with tumor cells (LNCAP). Figure shows the difference in white blood cell (WBC) numbers before a) and after b) CD45 depletion.
