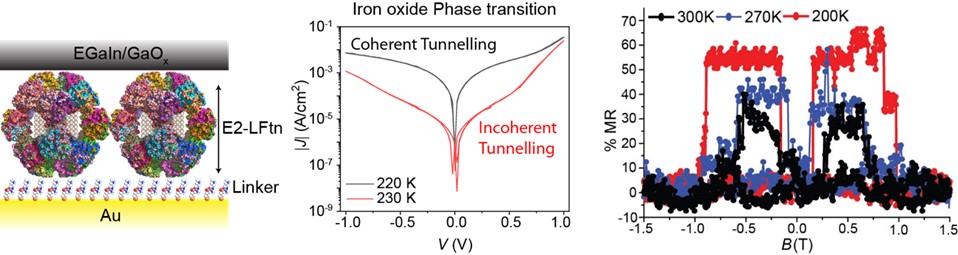
Countless number of natural processes (such as, respiration, photosynthesis, or DNA repair) involve large biomolecules that efficiently transport charges over large distances. Inspired by the efficiency at which Nature is capable of doing so, we study the mechanism of charge transport across biomolecules and biomolecular building blocks. We have been particularly interested in large globular proteins that are important in storing and the release of an iron ions. These proteins make it possible to study charge transport rates as function of the iron loading, quaternary structure of the protein, and temperature (the proteins are isolated from thermophiles and therefore stable over a broad range of temperatures). We found that ferritin is a particularly good conductor (small tunnelling decay coefficient; Adv. Mater. 2016, 28, 1824–1830). The figure below shows a junction with E2 (bioengineered with LFtn from ferritin) which has a large diameter of 25 nm in solution where the charge transport mechanism can be switched as a function of temperature (middle panel; J. Mater. Chem. C 2021, DOI: 10.1039/D0TC05773H). The panel on the right shows that junctions with ferritin loaded with iron oxide show large magnetoresistance even at room temperature (J. Phys.: Mater. 2021, 4, 035003).
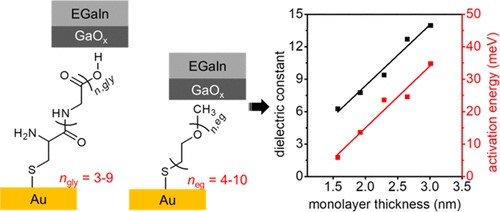
Various studies have shown that proteins conduct charge efficiently (low tunnelling decay coefficients). To shed more light on the reasons why charge transport is so efficient, we investigate junctions with SAMs of olioglycine. These SAMs have a surprisingly large dielectric constants (up to 14) which increases with molecular length (ACS Appl. Mater. Interfaces 2020, 12, 45111-45121). The activation energy (incoherent tunnelling rate) also increases with molecular length (figure below). These results indicate that charge transport in biomolecules cannot always simply be categorized as coherent vs incoherent tunnelling, and that alternative mechanisms have to be explored that may also be important or interesting in other areas of research.