CPM is involved in teaching both bachelor and master students in Chemical Science & Engineering. In addition, a course is given on kinetics and catalysis for the Process Technology course. In the following a short description of each course can be found including a reference to OSIRIS, the official course information system of the University of Twente.
For questions or additional information please contact Prof. Lefferts, Prof. Jimmy Faria Albanese or Dr. Aayan Banerjee
Course Overview
Course Module | Brief Name | Starting Block | Credits | Faculty |
|---|---|---|---|---|
Kinetics & Catalysis | 1A | 4.5 EC | TNW | |
Bachelor Assignment | JAAR | 15 EC | TNW | |
Advanced Catalysis | 1A | 5 EC | TNW | |
C.S. Catalytic Processes and Materials | JAAR | 5 EC | TNW | |
CR Assignment (Studytrip) | JAAR | 5 EC | TNW | |
MSc Final Project RGA | JAAR | 20 EC | TNW | |
MSc Final Project SRA | JAAR | 25 EC | TNW | |
MSc Final Project SRA | JAAR | 20 EC | TNW | |
Reaction Kinetics and Catalysis for PT | 1A | 4.5 EC | TNW |
Bachelor/Master Assignments
If you are interested in doing your bachelor or master assignment at CPM, please send an email to Prof. Leon Lefferts, Prof. Dr. Jimmy Faria Albanese, or Dr. Aayan Banerjee.
We will regularly update with new bachelor/master assignments, but also welcome new ideas.
BSc or MSc Assignment CPM: Development of Controlled Preparation Methods Bimetallic Heterogeneous Catalysts to be used in Liquid Phase Nitrate and Nitrate Reduction
Overview
Nitrate (NO3-) and nitrite (NO2-) are water contaminants that can cause human diseases such as blue baby syndrome when consumed with drinking water or which can lead to eutrophication in natural waters. NO3- and NO2- can be catalytically converted to N2 by heterogeneous metal catalysts consisting of e.g. palladium and a promotor metal such as tin, indium or copper.[1] Up until now, activity, selectivity and stability are insufficient for a commercial process. Therefore, we would like to fundamentally understand interaction of the two different catalyst metals to be able to prepare catalysts by design rather than by trial and error.
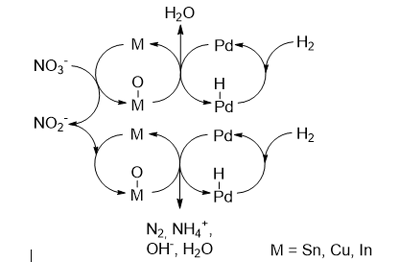
Figure 1: Proposed reaction pathway for bimetallic NO3- and NO2- reduction.
Heterogeneous catalysts typically consist of inorganic supports such as Al2O3 or SiO2 on which metal particles are deposited. Depending on the preparation method the microscopic metal structure and the metal-support interaction varies. In case of bimetallic catalysts, the two metals can form e.g. well mixed alloys, core-shell arrangements or separated particles.[2] For the nitrate reduction reaction close proximity or at least electron shuttle possibilities[3] are required for the regeneration of the Sn active sites (Figure 1). Therefore, it is of particular interest to prepare catalysts with close interaction of the metals. To do so, the chemical toolbox offers advanced catalyst preparation techniques such as co- or sequential strong electrostatic adsorption (co-/sq.-SEA),[4] charge enhanced dry impregnation (CEDI),[5] controlled surface deposition (CSD)[6] which exceed the potential of commonly applies dry- or wet impregnation preparations.[2]
Learning Objective
In your project, you will use advanced catalyst preparation techniques to prepare well controlled bimetallic catalyst with strong metal-metal interactions. Based on existing knowledge in our research group and your catalytic reaction results you will optimize the formulation of your catalysts and characterize them in depth. The catalytic tests will be conduced in batch and/or flow condition with subsequent ion chromatographic (IC) analysis. For the catalyst characterization XRF, XRD, CO-chemisorption, N2 physisorption, TPR/TPD are readily available in our Labs and STEM, EDS and ICP can be arranged.
Contact information
Daily Supervisor: Janek Betting (j.betting@utwente.nl)
Supervisor: Prof. Dr. Jimmy Faria Albanese (j.a.fariaalbanese@utwente.nl)
Literature
[1] I. Sanchis, E. Diaz, A. H. Pizarro, J. J. Rodriguez, A. F. Mohedano, Sep Purif Technol 2022, 290, 120750.
[2] B. A. T. Mehrabadi, S. Eskandari, U. Khan, R. D. White, J. R. Regalbuto, Adv. Catal. 2017,61, 1-35.
[3] K. M. Lodaya, B. Y. Tang, R. P. Bisbey, R. P. Bisbey, S. Weng, K. S. Westendorff, W. L. Toh, J. Ryu, Y. Roman-Leshkov, Y. Surendranath, An electrochemical approach for designing thermochemical bimetallic nitrate hydrogenation catalysts, Nat Catal 2024.
[4] A. Wong, Q. Liu, S. Griffin, A. Nicholls, J. R. Regalbuto, Science 2017, 358, 1427-1430.
[5] X. Zhu, H.-R. Cho, M. Pasupan, J. R. Regalbuto, ACS Catal. 2013, 3, 4, 625-630.
[6] A. Garron, K. Lázár, F. Epron, Appl Catal B 2005, 59, 57–69.
BSc Assignment CPM - Development of Pd-Pt/CeO2 Catalysts with Different Morphologies for Lean Methane Oxidation
Overview
Why a lean methane oxidation catalyst is needed?
These catalysts are needed to overcome the main problem of LNG-fueled ships, methane slip. The use of LNG-fueled instead of the standard MDO-fueled ships brings a reduction in emissions of harmful gases, such as SOx, NOx, and CO2, among others. [1] Nonetheless, the utilization of natural gas as a propellant leads to the release of unburnt methane, known as methane slip. Considering the substantially higher global warming potential of methane compared to CO2, [2] it is environmentally prudent to oxidize the unburnt methane into CO2. This necessitates the development of catalysts with high activity and stability in the presence of sulfur and water.
Why Pd-Pt/CeO2 nanorods, nano-octahedrons, and nanocubes?
Palladium (Pd) stands out as the most active metal in the context of lean methane oxidation oxidation. [3] Nevertheless, Pd is highly vulnerable to water and sulfur poisoning. To address this issue, studies suggest that the addition of platinum (Pt) may enhance resistance to water and sulfur poisoning. [4] Additionally, the inclusion of cerium dioxide (CeO2) has the potential to boost the concentration of oxygen vacancies, a crucial factor in oxidation reactions. Furthermore, tailoring different morphologies of CeO2 could further amplify its catalytic capabilities [5]

Picture a. Experimental Setup
Learning Objective
- Synthesizing novel Pd-Pt/CeO2 nanorods, nano-octahedrons, and nanocube catalysts, to derive state-of-the-art kinetics using the experimental setup equipped with online GC
- Learning and performing multiple types of catalyst characterizations
- Learning about advanced infrastructure to acquire experimental data and perform the characterizations
Contact information
Daily Supervisor: Martim Chiquetto Policano (m.chiquettopolicano@utwente.nl)
Supervisor: Prof. dr. Jimmy Faria Albanese (j.a.fariaalbanese@utwente.nl)
Literature
[1] Gélin & Primet, 2002; Hua et al., 2017
[2] Derwent, 2020
[3ab] a.Fujimoto et al., 1998; b.Gélin & Primet, 2002
[4] Nassiri et al., 2018; Sadokhina et al., 2018
[5] Sakpal & Lefferts, 2018
MSc Assignment CPM: Reactor Design for Lean Methane Oxidation on LNG Ships
Overview
Benefits that could arise from implementation of a lean methane oxidation reactor in LNG ships are a drastic reduction in the global warming potential. The use of LNG-fueled instead of the standard MDO-fueled ships brings a reduction in emissions of harmful gases, such as SOx, NOx, and CO2, among others. [1] Nonetheless, the utilization of natural gas as a propellant leads to the release of unburnt methane, known as methane slip. Considering the substantially higher global warming potential of methane compared to CO2, [2] it is environmentally prudent to oxidize the unburnt methane into CO2. This necessitates the development of catalysts with high activity and stability, along with the design of reactors, regeneration systems, and other requisite processes for successful implementation.

Picture a. Experimental Setup
Learning Objective
In collaboration with your supervisors, the experimental kinetic data for both novel and commercial catalysts will be obtained using the experimental setup illustrated in Figure 1a. Additionally, existing data from the literature, for example, [3] will be utilized for comparing various catalysts. These data will play a crucial role in modeling the reactor and other processes within the lean methane oxidation gas treatment unit. Simulations will be conducted to compare the operation under different catalysts and conditions, including varying concentrations of poisons such as SOx, [4] and water, [5] diverse temperatures, and different regeneration procedures.
Contact Information
Daily Supervisor: Martim Chiquetto Policano (m.chiquettopolicano@utwente.nl)
Supervisor: Prof. Dr. Jimmy Faria Albanese (j.a.fariaalbanese@utwente.nl)
Literature
[1] Gélin, P., & Primet, M. (2002). Complete oxidation of methane at low temperature over noble metal based catalysts: a review. Applied Catalysis B: Environmental, 39(1), 1–37. https://doi.org/10.1016/S0926-3373(02)00076-0
[2] Derwent, R. G. (2020). Global Warming Potential (GWP) for Methane: Monte Carlo Analysis of the Uncertainties in Global Tropospheric Model Predictions. Atmosphere, 11(5), 486. https://doi.org/10.3390/atmos11050486
[3] Habibi, A. H., Semagina, N., & Hayes, R. E. (2018). Kinetics of Low-Temperature Methane Oxidation over SiO2 -Encapsulated Bimetallic Pd–Pt Nanoparticles. Industrial & Engineering Chemistry Research, 57(24), 8160–8171. https://doi.org/10.1021/acs.iecr.8b01338
[4] Monai, M., Montini, T., Melchionna, M., Duchoň, T., Kúš, P., Chen, C., Tsud, N., Nasi, L., Prince, K. C., Veltruská, K., Matolín, V., Khader, M. M., Gorte, R. J., & Fornasiero, P. (2017). The effect of sulfur dioxide on the activity of hierarchical Pd-based catalysts in methane combustion. Applied Catalysis B: Environmental, 202, 72–83. https://doi.org/10.1016/j.apcatb.2016.09.016
[5] Mihai, O., Smedler, G., Nylén, U., Olofsson, M., & Olsson, L. (2017). The effect of water on methane oxidation over Pd/Al2O3 under lean, stoichiometric and rich conditions. Catalysis Science & Technology, 7(14), 3084–3096. https://doi.org/10.1039/C6CY02329K
Previous Projects
Below you will find an overview of some of the previous projects done by bachelor and master students at CPM in no particular order.
- Catalyzed Carbonization of Biomass
- Effect of Morphology of Support on Stability of Cu/CO2 Catalyist for CO2 Hydrogenation to Methanol
- Performance Analysis of a Newnano-Cell Design for High Throughput Low-Temperature Electrolysis
- Fundamental Studies of Nitrites Diffusion on Functionalized Membranes with Stimulus Responsive Polymers using ATR-IR
- Effects of Applying Plasma for an Electrified Steam Methane Reforming Process
- Two-Stage Plasma-Catalytic Ammonia Synthesis over Pd/MgO Catalysts
- Effect of Polymer Brush Growth and Catalyst Support Size on Metal Catalyzed Nitrite Hydrogenation
- Plasma-Based NOX Synthesis with In-situ Adsorption
- Modelling the Effect of a Density Profile on Low-Fouling Polyelectrolyte Brushes for Sensing applications
- Direct Synthesis of Formaldehyde from Syngas using Plasma-Catalysis
- Reaction Pathways in the Dehydration of Propyleneglycol over Scandiumoxide Catalyst
- Support Effects on Plasma-Catalytic Ammonia Ssynthesis over Cobalt-Based Catalysts
- Kinetic Modelling of Polymer-Modified Metal-Supported Catalysts for Nitrite Hydrogenation Reactions
- High Selective Catalyst Based on Pd Nanoparticles Loaded on Hydrogel for Nitrite Hydrogenation
- The Investigation into Catalyst Stability of Co-Processing of Fast Pyrolysis Bio-Oil with the Fluid Catalytic Cracking Process
