The physics of ions in liquid are directly relevant to a surprisingly wide array of research areas of current scientific and societal interest. These include nanoscience (the ‘natural’ length scale for ions), energy (fuel cells, supercapacitors), neuroscience (signal transduction, new experimental tools), and health and environment monitoring (new and better sensors).
Our research is currently divided into a number of distinct but connected lines:
1.SINGLE CONDUCTING POLYMERS:
We are exploring a new eletronic transduction mechanism based on the detection of water-solutble conducting polymers. Despite their wide usage in the form of chemically doped conducting films, surprisingly little is known about the intrinsic electronic transport properties of these macromolecules in their native state in solution as fluctuating random coils. In the fundamentally oriented aspect of the project, we employ pairs of electrodes separated by ca. 10 nm to probe both the DC, AC and noise properties of conduction through these materials. On the applied side of this research, we carry out proof-of-concept demonstrations of electrical signal transduction for biosensing at the single-molecule level.
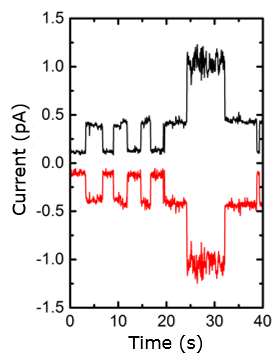
2.Electrochemical nanofluidics:
We employ micro/nanofabrication to create liquid-filled, nanometer-scale channels and chambers in which small numbers of molecules (and even single molecules) are detected and manipulated using electrical signals. Our devices count among the most sensitive electrochemical sensors built to date. In collaboration with colleagues at the UT, we are currently extending this technique to allow the detection of tracer amounts of DNA cancer markers in bodily fluids such as urine.

3.High-frequency CMOS nanocapacitor array sensors:
Massively parallel, label free biosensing platforms can in principle be realized by combining all-electrical detection with low-cost integrated circuits. We explore the physics underlying the operation of large-scale, high-density array of nanoelectrodes integrated with CMOS electronics on a single chip. This approach allows detecting and fingerprinting analytes ranging from viruses and inorganic nanoparticles to living cells.