Redox cycling at nanospaced electrodes
Towards electrochemically amplified biomolecular sensing
Dr. M.J.J. van Megen
Molecule synthesis is convenient on the small scale while electrode fabrication is more convenient on the large scale. In this project they meet each other halfway for the development of a twin electrode design featuring surface attached molecules undergoing redox cycling. Research has focused on novel applications of the redox cycling phenomena, fabrication of electrodes with an inter electrode spacing in the nanometer range, and the electrochemical behavior of surface attached ferrocene labeled polyethylene glycol (PEG). This knowledge is combined in experiments where the PEG molecules are cycling between two electrodes spaced < 100nm apart. This setup can be used as the transducing element of an electrochemical sensor if the molecules are labeled with an additional recognition element.
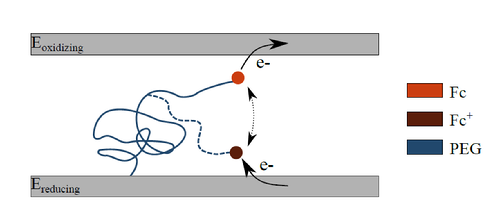
Illustration of the RC concept using surface attached redox labeled molecules. The PEG molecule is large enough to reach both of the electrodes with its ferrocene (Fc) redox label. As a result, the ferrocene can undergo repeated oxidation and reduction reactions, thereby shuttling electrons between the two electrodes.
