Aim is to:
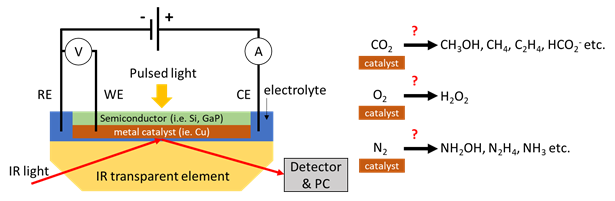
The goal of our research is to fully understand how CO2 (or other feedstock) is being converted to valuable products like methane or ethanol at the electrode of an electrolyser or photoelectrochemical cell. This will guide catalyst design for increasing device efficiency and improving product selectivity that, ultimately, enables scaling up green production of fuels to impactful levels. To be able to uncover which and how intermediates are being formed at the electrode (and what the kinetics look like!), we deploy a new powerful approach utilizing time-resolved Infrared spectro-electrochemistry in-situ.
Spectroscopic technique: polarized FT-IRRAS, ATR FT-IR, SEIRAS, Electrochemistry
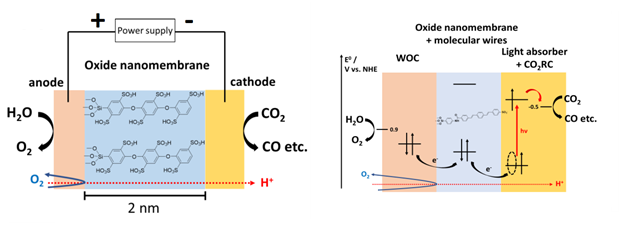
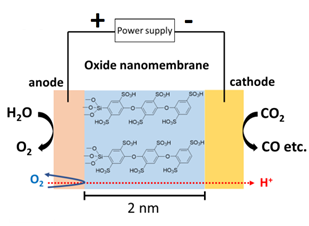
Ultrathin oxide layers as membranes for photo- and electrocatalysis
Ultrathin oxide layers open up new pathways for the hierarchical integration of electro- or photocatalysts, and membranes. Inspired by Nature, these nanomembranes can embed functional molecules to tune reactant impermeability, proton transport, and electron conductivity for cross-coupling of catalysts. This will minimize efficiency degrading processes such as long mass transport path lengths and chemical side reactions. We are studying structure and electron & proton transfer dynamics of such molecule embedded nanomembranes to separate the incompatible catalysis environments on the nano-scale. Anode/nanomembrane/cathode nano assemblies will be integrated into macro array systems.
Techniques: nanofabrication, sputtering, ALD, PLD, anodization, templated synthesis, molecular assembly, electrochemistry, impedance spectroscopy, catalysis
- Dr.Ir. G. Katsoukis - TNW-PCS Group University Twente
Recent Publications:
- The project has recently initiated
