Tuning the selectivity of aldehyde reduction
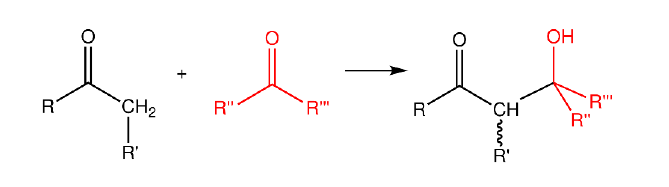
The electrochemical synthesis of organic compounds of commercial relevance from biomass-derivable feedstock is a renewable alternative to existing petrochemical-based synthesis pathways. Furthermore, it is possible for carbonyl compounds to condense to form longer chain carbohydrates1, hence, to produce artificial sugars in a renewable fashion.
However, unlike many inorganic reactants, organic molecules can undergo a vast number of electrochemical transformations leading to poor yields which further complicate the downstream processing required to obtain purified fractions2. This is further complicated by the fact that several key aqueous organic reactions occur at potentials outside the narrow electrochemical stability window of water leading to competition with the hydrogen or oxygen evolution reactions.
It has been shown that both aldehyde reduction and aldol condensation are particularly sensitive to the pH of the electrolyte3. This offers a way to ‘tune’ the reaction by carefully controlling the pH, and particularly the local pH at the electrode/electrolyte interface.
This master assignment focuses on designing an efficient reaction scheme by tuning the pH in one or more steps to achieve optimal production of aldol condensates. This project, involving industrial consortium partners (Nobian Chemicals B.V. & Biomass Technology Group BTG B.V.), will involve the use of analytical methods like gas chromatography and liquid chromatography to analyze product distributions. The experimental campaign will be designed to understand the influence of the electrolyte pH on the reaction kinetics, hence, to identify suitable electrochemical process conditions leading to high yields of desired products. More on the project? See this press release!
Contacts
Dr. Akash Raman, Postdoctoral researcher, a.raman@utwente.nl
Prof. Marco Altomare, m.altomare@utwente.nl
Dept. Chemical Engineering, MESA+ Institute for Nanotechnology, University of Twente
References
1. Omran, A., Menor-Salvan, C., Springsteen, G., and Pasek, M. (2020). The Messy Alkaline Formose Reaction and Its Link to Metabolism. Life 10, 125. https://doi.org/10.3390/life10080125.
2. Balcells, D., Clot, E., Eisenstein, O., Nova, A., and Perrin, L. (2016). Deciphering Selectivity in Organic Reactions: A Multifaceted Problem. Acc. Chem. Res. 49, 1070–1078. https://doi.org/10.1021/acs.accounts.6b00099.
3. Sousa, L.F. de (2022). Electrochemical valorization of sustainable feedstocks using metal-based gas diffusion electrodes. https://doi.org/10.3990/1.9789036554879.
