Performance and stability of cocatalyst nanoparticle-modified SrTiO3 single crystals as model photocatalysts for water splitting and green hydrogen
Climate change stands out as one of the major issues of the 21st century. Its primary driver is global warming, which is predominantly caused by excessive emissions of greenhouse gases, particularly from the transportation, energy, and industry sectors. Hydrogen emerges as a promising alternative to fossil fuels, due to its high energy density and zero carbon emission upon combustion. However, currently, more than 95% of the used hydrogen is produced from fossil fuels. Therefore, there is a strong environmental and societal push to replace fossil fuel-based energy sources with more sustainable alternatives such as hydrogen. Besides electrochemical technologies, such as water electrolysis, it is also envisioned that hydrogen could be produced by photocatalytic water splitting.
Photocatalysis, in general, relies on the use of semiconductors with a bandgap suitable to harvest the energy of light, ideally solar radiation. Semiconductor photocatalysts enable charge carrier generation upon bandgap excitation. Once electron-hole pairs are generated, they can diffuse from the bulk to the surface of the semiconductor, where they can drive surface redox reactions according to the potential of the bandgap edges, that is, according to the electrochemical potential of valence band holes (VB h+) and conduction band electrons (CB e–).
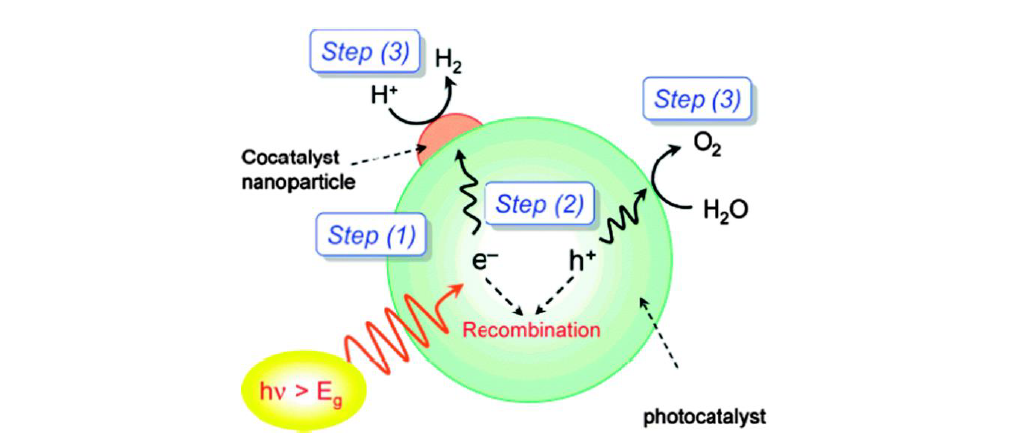
Sketch of a photocatalyst particle under illumination and bandgap excitation (from J. Phys. Chem. C 2007, 111, 22, 7851, https://doi.org/10.1021/jp070911w )
Cocatalyst nanoparticle (NP) modified semiconductor, such as metal modified-SrTiO3 (STO) – as shown in the sketch above – are suitable candidate for photocatalytic water splitting since they show promising performance. However, they suffer from degradation in aqueous media under irradiation, particularly when using earth abundant metal cocatalyst materials. Such degradation causes losses in activity during photocatalysis.
In Recent work from our PCS group,[1] the deactivation mechanism of a Ni-STO was analyzed by employing in situ X-ray absorption spectroscopy (XAS) techniques. The results show that the instability of the Ni cocatalyst derives from its oxidation and reconstruction into inactive Ni(II) species. These redox processes are induced by photogenerated valence band holes in the illuminated photocatalyst. This study was performed with powder photocatalysts tested in aqueous suspensions (slurries). Such catalysts consist of STO powders surface-modified with Ni NPs, where Ni and STO particles typically feature inhomogeneous size, and the Ni NPs are randomly distributed on the STO surface. Thus, to investigate further the performance and stability of cocatalyst modified STO photocatalysts for water
2
splitting, this master thesis project will deal with synthesizing and testing a model metal NP-STO photocatalysts featuring defined morphology, structure, and composition. For this, you will use high-end deposition nanotechnologies including physical vapor deposition (PVD, sputtering) and controlled thermal treatments (solid-state dewetting). Precisely, you will use STO photocatalyst supports in the form of single crystals (SC) and deposit metal cocatalyst NPs by solid-state dewetting (see figure below) – this involves the heat treatment of STO single crystals coated with thin metal cocatalyst films (e.g., a few nm-thick) under controlled temperature and gas atmosphere, which will cause the agglomeration of the metal cocatalyst film into defined metal NPs[2,3] directly at the STO semiconductor surface.

(Top) Sketch of the fabrication and (Bottom) SEM images of an example of defined metal NP-modified STO photocatalyst (in this case, Ni-STO; unpublished results).
Your work aims to analyze the performance and stability of metal-STO photocatalysts for water splitting. You will achieve this goal by analyzing the H2 generation performance in photocatalytic tests with our continuously stirred reactor coupled with online gas chromatography. You will evaluate the photocatalyst stability also using techniques such as UV-Vis diffuse reflectance, XRD, SEM, TEM and XPS.
Contacts
In case you have any questions, feel free to contact MemetTursun Abudukade and Marco Altomare Daily supervisor: MemetTursun Abudukade (Meander Room ME149) m.r.abudukade@utwente.nl Supervisor: Prof. Marco Altomare m.altomare@utwente.nl
References
[1] M. T. Abudukade, M. Pinna, D. Spanu, G. De Amicis, A. Minguzzi, A. Vertova, S. Recchia, P. Ghigna, G. Mul, M. Altomare, Journal of Physical Chemistry C 2024, 128, 11.
[2] C. V. Thompson, Annu Rev Mater Res 2012, 42, 399–434.
[3] M. Altomare, N. T. Nguyen, P. Schmuki, Chem Sci 2016, 7, 6865–6886.
