MSc assignment: coupled electrolysis of CO2 reduction and glucose oxidation
Supervisors: Kasper Wenderich (k.wenderich@utwente.nl)/Shri Kannan Chandrasekar (s.k.chandrasekar@utwente.nl)/ Bastian Mei (b.t.mei@utwente.nl)
Background
The Paris agreement signed in 2015 to battle climate change aims to bring the global average temperature to below 2˚C of the pre-industrial levels. This necessiates the reduction in greenhouse gas emissions, carbon dioxide in particular, the major greenhouse gas from anthropogenic activities. Using electrochemistry to convert CO2 to syngas (CO + H2) and/or hydrocarbons has gained great attention over the last years [1]. This in combination with renewable electricity to drive electrocatalytic reactions makes the prospect extremely attractive for industry. Moreover, recent research demonstrates long term stability of electrochemical CO2 conversion at high current densities [2, 3]. However, the high cost and the scarcity of the electrode material would be a stumbling block in the industrial adoption of this process.
Here at PCS, we’ve established an electrochemical CO2 reactor setup where we studied alternative anode materials to the commonly used iridium which is scarce and expensive. Now, we would like to replace the usual oxygen evolution reaction (which has a low market value) with a reaction with more commercial attractiveness [4]. Specifically, the electrochemical upgrading of biomass is of interest [5-7], as underlined in 2004 by the US Department of Energy (DOE). Glucaric acid has a market value (550.4 million USD in 2016). It serves as a building block for many industrial applications (such as polymers and nylons), and most importantly, it is suggested as a replacement of harmful phosphates used for the fabrication of detergents. Glucaric acid can electrochemically be synthesized through the oxidation of glucose, with the oxidation of glucose to gluconic acid as intermediate step. Also gluconic acid is an important added value product, as it finds applications in the food industry and the synthesis of pharmaceutical and hygeniec products. In this project, we aim to selectively oxidize glucose to gluconic acid, and subsequently from gluconic acid to glucaric acid.
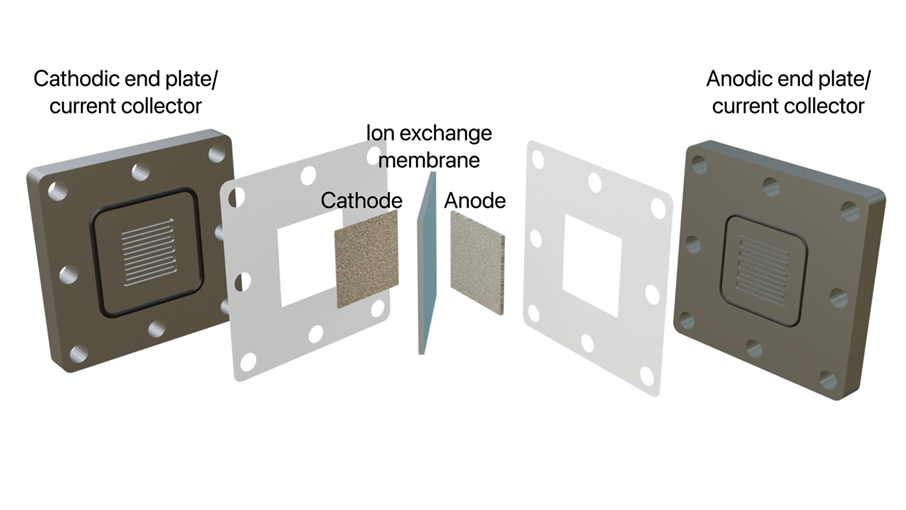
Figure 1. Schematic depicting the envisioned electrochemical reactor that will be used during the study.
Goal
In this project, the student will work on testing a device to succesfully perform the coupled
electrolysis for CO2 reduction to CO and glucose to gluconic acid. The project will consist out of two steps:
1. First, the student will familiarize themselves with the electrochemical oxidation of glucose to gluconic acid. In our group, we have set up a ‘standard’ electrochemical flow cell suited to do this. The anode consists of gold, which is typically well-suited for the oxidation of glucose under alkaline conditions [6]. The student will study the electrochemical properties of glucose oxidation under alkaline conditions. By using an HPLC and a GC, the student will analyze what the optimal conditions (e.g., applied voltage or electrolyte composition) are to form gluconic acid at high efficiencies and with high production rates.
2. Subsequently, the student will fabricate and use membrane electrode assemblies to study the coupled electrolysis of CO2 to CO and glucose to gluconic acid. The integration of a suitable anode is of key importance and might be realized through electrodeposition on a suitable porous support. Also here, the student will optimize reactor operation to enable paired-electrolysis at current densities of industrial relevenace.
References
[1] Burdyny, T. & Smith, W.A.; CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environmental Science
2019, 12, 1442-1453
[2] Endrődi, B. et al.; Operando cathode activation with alkali metal cations for high current density operation of water-fed zero-gap carbon dioxide electrolysers. Nature Energy 2021, 6, 439-448
[3] Liu, Z. et al.; CO2 Electrolysis to CO and O2 at High Selectivity, Stability and Efficiency Using Sustainion Membranes. Journal of The Electrochemical Society 2018, 165 (15), J3371-J3377
[4] Mei, B.T. et al.; Beyond water splitting: efficiencies of photo-electrochemical devices producing hydrogen and valuable oxidation products. Advanced Sustainable Systems 2017, 1, 1600035
[5] Liu, W.-J. et al.; Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nature Communications 2020, 11:265
[6] Moggia, G. et al.; Two-steps synthesis of D-glucaric acid via D-gluconic acid by electrocatalytic oxidation of D-glucose on gold electrode: Influence of operational parameters. Electrochimica Acta 2021, 374, 137852
[7] Schlegel, N. et al.; On the electrooxidation of glucose on gold: Towards an electrochemical
glucaric acid production as value-added chemical. Electrochimica Acta 2022, 410, 140023
