Scaling Up Hydrothermal Synthesis of TiO₂ Nanoparticles for Photothermal CO₂
Reduction
Are you excited about nanomaterials, catalysis, and sustainable energy conversion technology? Do you want to work on a project that combines chemistry, materials science, and renewable energy? Join us in developing facet-controlled TiO₂ nanoparticles to take photothermal CO₂ reduction to the next level.
Project Overview
Rising CO₂ emissions contribute to various environmental problems. But what if we could convert CO₂ into valuable products like methane (CH₄)? By harnessing light energy, we can convert CO₂ in a sustainable way. Even better, if we combine light and heat energy, the process becomes even more efficient. This approach—photothermal CO₂ reduction (figure 1)—offers a promising route toward cleaner energy and carbon utilization.

Figure 1: Photothermal CO2 reduction to CH4.
In nature, materials are structured at the nanoscale to achieve remarkable efficiencies. Hydrothermal synthesis is a method that allows us to control the shape, size, and facets of nanomaterials. By optimizing and scaling up this technique, we can enhance TiO₂ nanoparticles' catalytic properties and improve their performance in photothermal CO₂ reduction reactions, as shown in figure 2. In this project, your goal will be to perform photothermal CO2 reduction on Ru deposited on facetted TiO2, as shown in figure 2.
What will you learn from this project?
> Scale up hydrothermal synthesis – Learn how key factors influence nanoparticle growth and optimize them for large-scale production.
> Master advanced characterization techniques – Use SEM, TEM, XPS, AFM, DRS, and XRD to analyze material properties at the nanoscale.
> Explore reaction mechanisms – Utilize in situ DRIFTS spectroscopy to monitor molecular interactions during CO₂ reduction.
> Enhance catalytic performance – Deposit ruthenium (Ru) or other metal nanoparticles using techniques like photo-deposition and colloidal deposition.
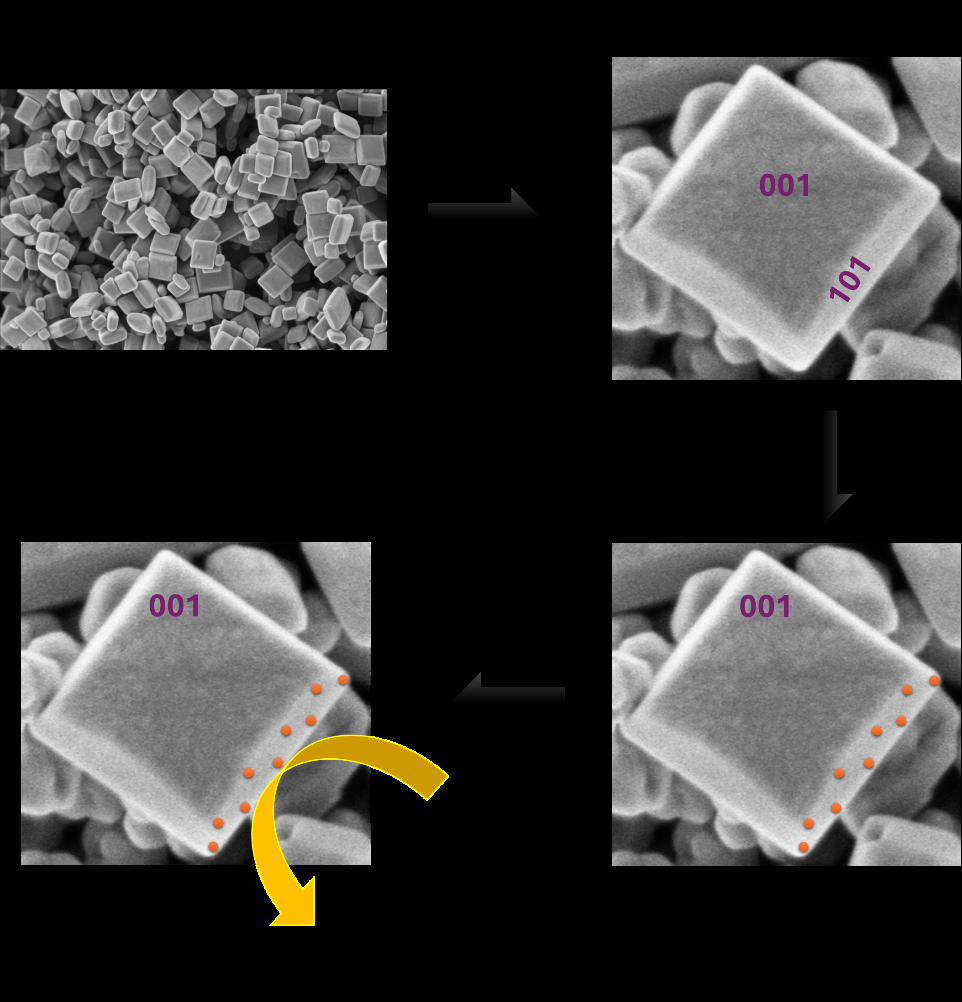
Figure 2: CO2 reduction to CH4 on Ru deposited facetted TiO2 nanoparticles
Why should you apply for this BSc or MSc assignment?
🚀 Work on a high-impact project at the intersection of materials science, catalysis, and
sustainable chemistry.
🔬 Gain hands-on lab experience in synthesis, characterization, and reaction analysis—
valuable skills for careers in nanotechnology, energy, and chemical engineering.
🌍 Contribute to cutting-edge research that tackles one of the biggest global challenges: carbon capture and utilization.
Supervision
You will be closely mentored by Anuradha Meena and Dr. Ir. Kasper Wenderich from
the Photocatalysis Synthesis Group in the Department of Chemical Engineering.
📩 Interested? Have questions?
Contact us at:
✉ anuradha.meena@utwente.nl
✉ k.wenderich@utwente.nl
