Master assignment: '' The role of aprotic ionic liquids for electrochemical CO2 conversion"
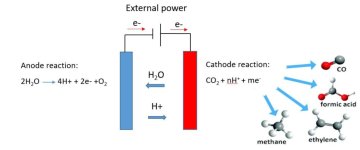
CO2 capture from air and its conversion to value added products like fuels can close a cycle with which zero-net emission technology will be realized. When captured in a solvent, CO2 can be converted to a fuel by means of electrochemical conversion (Scheme 1). There CO2 conversion occurs at the cathode electrode and water oxidation precedes at the anode electrode. Depending on the type of electrode and electrolyte the products of CO2 conversion can be syngas (CO and H2), formic acid and other hydrocarbons. While very promising, CO2 conversion still suffers from some problems which need to be overcome before industrial trial. For CO2 reduction to happen, in a first stage, one electron should be transferred to CO2 to form CO2 radical (CO2-) [1]. This intermediate is very unstable and requires high activation energy which in turn results in sluggish kinetics for overall reaction. Hydrogen evolution reaction (HER) competes with CO2 reduction at the cathode electrode and this decreases the selectivity of CO2 reduction. Room temperature ionic liquids (molten salts) have been shown to mitigate these problems with co-catalytic effects [2]. Unfortunately there is no decisive conclusion regarding the origin of these promoting effects [3]. In this master assignment, on the basis of chemical variations including: hydrogen bonding abilities, acidity and basicity; a series of aprotic ionic liquids will be examined as co-catalyst for CO2 reduction. The effects of selected ionic liquids for CO2 conversion will be investigated at gold and silver electrodes which are relevant for CO production. This systematic investigation could help for a more clear picture from the role of ionic liquids which in turn will help to design or select ionic liquids with desired performance for CO2 conversion.
Sources:
- Y.Hori, "Electrochemical CO2 Reduction on Metal Electrodes", in Modern aspects of electrochemistry, By Constantinos.G. Vayenas. No: 42, 89-189.
- Brian A. Rosen, et al, "Ionic Liquid–Mediated Selective Conversion of CO2 to CO at Low Overpotentials", Science, 334, 643-644. (2011)
- H. Lim and H. Kim, "The Mechanism of Room-Temperature Ionic-Liquid-Based Electrochemical CO2 Reduction: A Review", Molecules, 22, 536. (2017)
