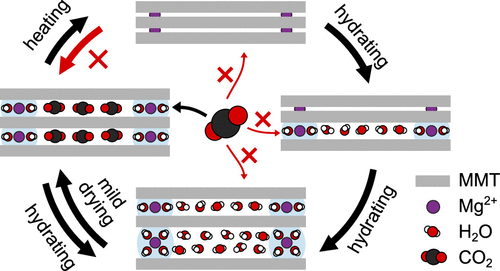
Layered swelling clay minerals like montmorillonite (MMT) can competitively and synergistically adsorb CO2 and H2O in their interlayer galleries. The general motivation of the present study arises from our goal to use smectites as a sorbent to separate CO2 from mixed gas streams (like biogas) that often contain finite amounts of humidity. This work examines how different interlayer cations, relative humidity levels (and amount of cosorbed H2O), and (de)hydration history affect CO2 adsorption on MMT and MMT-rich bentonite at near-ambient pressure and temperature. For CO2 to be adsorbed, the MMT requires either large (e.g., Cs+) or hydrated interlayer cations to provide a sufficiently wide interlayer gallery, and must not have too much H2O adsorbed competitively with CO2. Na-MMT and initially anhydrous Mg- and Ca-MMT studied under increasing relative humidity conditions adsorb little CO2. However, Mg- and Ca-MMT can effectively adsorb CO2 if first hydrated and then mildly dried such that the cations remain hydrated while the competitively adsorbed excess H2O is removed. Because of their high stability and the favorable shape of their CO2 adsorption isotherms, low-cost (near-)natural Mg- and Ca-bentonite can be used for (cyclic) CO2 adsorption and separation purposes, similar to the more expensive Cs-bentonite.