Understanding and overcoming passivating Fe(II,III)-rich Surface Alteration Layers in olivine dissolution
Overview
Capturing and storing CO2 from the atmosphere to mitigate global warming is one of humanity's most pressing challenges in the coming decades. One promising approach is the mineralization of CO2 using olivine, a magnesium-iron silicate mineral. The efficiency of olivine dissolution, however, is hindered by the formation of surface alteration layers (SALs) 1, which can impede the transport of bulk solid species into the reactive fluid. When iron-rich species are present, they can greatly slow down olivine dissolution even up to a complete halt 2. The formation of Fe (II, III) oxide species within the SAL occurs under conditions where pH, fluid composition, and oxygen pressure favor iron oxidation and reprecipitation 3. The properties of these Fe-rich SALs, however, remain poorly understood. Gaining a deeper understanding of their formation and evolution of these SALs is essential for developing strategies to enhance the efficiency of olivine dissolution, ultimately improving CO2 mineralization.
Research objective
The goal of this MSc project is to study the composition and evolution of the reprecipitated iron-rich species during olivine dissolution. The distribution of the oxides within the layer and their structure will be characterized with ex-situ and in-situ Confocal Raman Microscopy (CRM). Additional techniques like Scanning Electrons (SEM), Transmission Electrons (TEM) microscopies, powder X-Ray Diffraction (XRD) and X-Ray Photoelectron Spectroscopy (XPS) could be used to further understand the layer formation. The degree of passivation of the iron oxide coatings will be explored and correlated to the reactive environment and architecture of the SAL.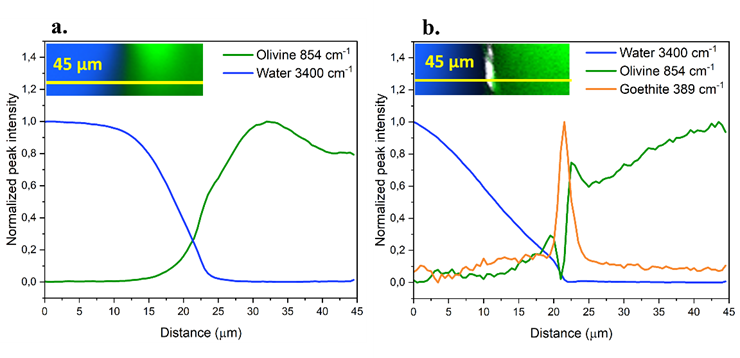
Figure 1 - CRM intensity profiles in a water-olivine system (a.) before and (b.) after 28 days of dissolution, when a Fe(III)-rich SAL has formed.
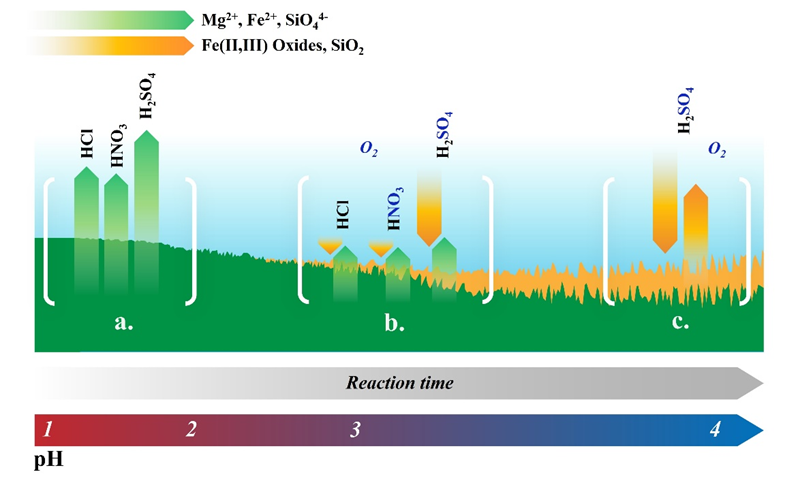
Figure 2 – Representation of the proposed process for the formation of Fe(II,III)-rich alteration layers in an olivine dissolution reaction. At pH values from 1 to ~2.5 (a.), olivine develops no Fe(III)-rich layer. From pH ~2.5 to ~3.5 (b.), an inhomogeneous Fe(III)-rich layer appears.
Learning objectives
In addition to the standard learning objectives for a master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
· Learn about dissolution-reprecipitation reactions and their applications in CO2 capture.
· Learn about in-situ characterization techniques;
· Hands-on experience on Confocal Raman Microscopy and several other microscopy and
spectroscopy techniques;
· Explore the potential applications of hematite in water splitting photocatalytic reactions 4;
· Learn about useful data processing techniques on hyperspectral images (Denoising, Principal
Component Analysis, Clustering);
· Develop experience in MATLAB and/or Python.
Contact information
· Daily Supervision: MSc. Vincenzo Alagia
· Supervision: Prof. Michael H.G. Duits
Literature
(1) Oelkers, E. H.; Declercq, J.; Saldi, G. D.; Gislason, S. R.; Schott, J. Olivine dissolution rates: A critical review. Chemical Geology 2018, (500), 1-19. DOI: 10.1016/j.chemgeo.2018.10.008.
(2) Saldi, G. D.; Daval, D.; Guo, H.; Guyot, F.; Bernard, S.; Le Guillou, C.; Davis, J. A.; Knauss, K. G. Mineralogical evolution of Fe-Si-rich layers at the olivine-water interface during carbonation reactions. American Mineralogist 2015, 100 (11-12), 2655-2669. DOI: 10.2138/am-2015-5340.
(3) Knafelc, J.; Filiberto, J.; Ferré, E. C.; Conder, J. A.; Costello, L.; Crandall, J. R.; Dyar, M. D.; Friedman, S. A.; Hummer, D. R.; Schwenzer, S. P. The effect of oxidation on the mineralogy and magnetic properties of olivine. American Mineralogist 2019, 104 (5), 694-702. DOI: 10.2138/am-2019-6829.
(4) Wang, D.; Chang, G.; Zhang, Y.; Chao, J.; Yang, J.; Su, S.; Wang, L.; Fan, C.; Wang, L. Hierarchical three-dimensional branched hematite nanorod arrays with enhanced mid-visible light absorption for high-efficiency photoelectrochemical water splitting. Nanoscale 2016, 8 (25), 12697-12701, 10.1039/C6NR03855G. DOI: 10.1039/C6NR03855G.
