Overview
Dilute concentrated electrolyte solutions can be understood in terms of classical continuum theories where ions are considered as point particles characterized solely by their charge density, immersed in a continuum dielectric fluid such as water. In this regime, the Poisson-Boltzmann theory gives a good description of ion distributions and the Nernst-Planck equation describes transport properties. Modern applications for energy storage, however, require electrolytes with ultrahigh ion concentrations to maximize the capacity of batteries. In this regime, classical continuum theories fail and specific ion effects as well as correlations between ions and ions and solvent molecules need to be accounted for.
One spectacular deviation from the classical behavior is the so-called anomalous underscreening. According to a series of reports in recent years, the ability of electrolytes to screen electrostatic charges decreases with increasing salt concentration in the range of very high concentrations leading to an increase in the decay length of electrostatic forces. This is in contrast to the conventional opposite trend of a decreasing Debye length (see figure) that is well established at lower concentrations and stimulated intense theoretical investigations. However, careful Atomic Force Microscopy experiments at the Physics of Complex Fluids group could not reproduce the phenomenon. Whether anomalous underscreening is a general ubiquitous phenomenon as originally claimed or whether it requires very specific conditions is therefore currently a matter open debate that needs to be resolved.
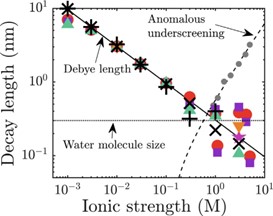
Experimentally observed and theoretically predicted decay length of electrostatic forces versus salt concentration. (from [1])
Research Objective
The goal of this Master assignment is to shed light into the question discussed above and – more generally – on the properties of highly concentrated aqueous salt solutions in general. For the highest possible concentrations of specific salts such as CsF, the number of ions per unit volume is similar to the number of solvent molecules. This makes the ‘solution’ a very peculiar fluid. You will perform Atomic Force Microscopy experiments using these solutions to study both ‘long range’ electrostatic forces as well as short range correlations on the level of the molecular size. You will explore different substrates that provide different boundary conditions for the ions and you may explore the use of an external bias voltage to control the adsorption of ions at the interface.
Learning Objective
In addition to the standard learning objectives for a master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
· Learn to perform and interpret high resolution Atomic Force Microscopy experiments
· Learn about aqueous electrolyte and their properties (which are ubiquitous in nature and technology)
· Have basic chemical-lab training (preparing solutions and surfaces, etc.)
· Depending on your interest, you may write analysis code in Python or Matlab and possibly develop a 3D surface solvation mapping routine
Contact Information
· Daily Supervision: Dr. Igor Siretanu (i.siretanu@utwente.nl)
· Supervision: Prof. Dr. Frieder Mugele (f.mugele@utwente.nl)
Literature
[1]: S. Kumar et al., Journal of Colloid and Interface Science 622 (2022) 819–827
