Overview
Capturing and sequestering CO2 from the atmosphere to mitigate global warming is one of the biggest challenges for mankind in the decades to come. Mineralization of CO2 using olivine, a magnesium iron silicate, is a promising candidate process be it through natural weathering or industrial conversion. However, the formation of a silica-rich surface alteration layer (SAL) is reported to drastically slow down the exchange of ions with the ambient medium. Both ‘leaching’ and ‘reprecipitation’ have been proposed as mechanistic explanations for SAL formation. At PCF, we work on elucidating the micro-scopic origins by using a combination of high resolution techniques including Fluorescence Lifetime Imaging (FLIM), Confocal Raman (CRM), Atomic Force (AFM) and Scanning Electron (SEM) microscopies.
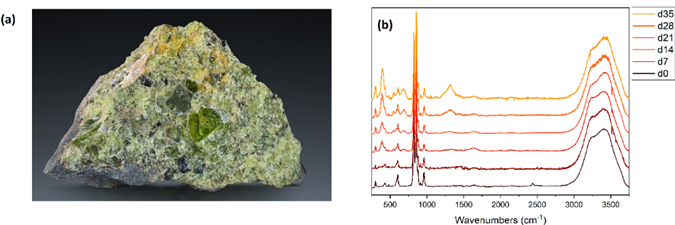
a) Typical aggregate of an olivine grain. b) Evolution of the Raman spectrum of an olivine pebble dissolved in H2SO4 at pH 2. Strong peaks emerge after 21 days
Research objective
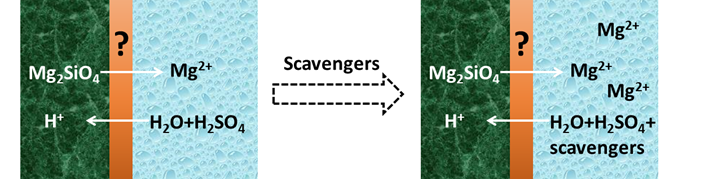
The main goal of the MSc project is to study the effects of cation and anion ‘scavengers’ [1,3] on the olivine dissolution rate and the SAL formation. Scavengers can facilitate the detachment of ions from the dissolving mineral and/or hinder reprecipitation of a new solid. We will measure this via the released Mg2+ ions, using FLIM with a Mg-responsive dye. After finding the right additive, we want to optimize its concentration. The additives’ effect on surface morphology will be studied with AFM and SEM, while changes in the chemical composition of the Surface Alteration Layer are studied with CRM.
Learning objective
In addition to the standard learning objectives for a master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
· Acquire knowledge on mineral carbonation techniques, including dissolution-reprecipitation reactions, and their practical applications in CO2 capture.
· Gain experience in Fluorescence Lifetime Imaging Microscopy (FLIM) and data analysis.
· Learn about high resolution Raman spectroscopy and data analysis.
· Use / develop experience in MATLAB and Python (data analysis)
Contact information
· Daily Supervision: Dr. Shilpa Mohanakumar (s.mohanakumar@utwente.nl)
· Supervision: Prof. Dr. Frieder Mugele (f.mugele@utwente.nl)
LIterature
[1] Oelkers et al., Olivine Dissolution Rates: A Critical Review. Chem. Geol., 2018, 500, 1-19
[2] Daval et al., Influence of amorphous silica layer formation on the dissolution rate of olivine at 90 °C
and elevated pCO2, Chem. Geol., 2011, 284, 193-209.
[3] Olsen et al, Oxalate-promoted forsterite dissolution at low pH, Geochim. Cosmochim. Acta, 2008,
72, 1758-66
