BACKGROUND
Polar materials are a class of materials with a switchable electrical polarization that can affect surface stoichiometry, surface chemistry, the electronic structure of the surface and the bulk of the material. Furthermore, due to their persistent response to electric fields, ferroelectrics, a special class of polar materials, offer a unique opportunity to tune the properties of a surface via an external field. These properties make polar/ferroelectric materials very interesting for electrocatalysis as they potentially could be used to enable a new level of control over the surface of a catalyst during catalysis.[1]–[3]. Based on this idea various authors have reported different cases of experimental and theoretical work on enhancement of the OER in electrochemistry by using polar/ferroelectric catalysts. However, no clear mechanism for ferroelectric OER enhancement or design rules for polar/ferroelectric OER catalyst purposes have been found. Our challenge is to find a clear correlation between activity and local polar/ferroelectric properties in OER catalysts such that a better understanding of the possible use of ferroelectric materials for OER catalysis can be obtained. We propose the use of epitaxial thin film oxides as model systems to experimentally investigate correlation. These pulsed laser deposition (PLD) grown well-defined epitaxial thin catalytic films allow us to carefully tune the polar properties of the material to try to differentiate magnetic induced effects from effects induced by its other properties. Moreover, as the surface chemistry, structure and charge are hypothesized to be the key parameters in understanding the correlation between polarity and OER activity [2,4,5,6] local probes of these parameters under OER conditions are identified as ideal candidates for studying polar/ferroelectric model systems. [7]
In this master thesis we will use a dual-scale Atomic Force Microscopy (ds-AFM) method that allows the determination of local surface potentials with a lateral resolution of ≤10nm in combination with atomic resolution imaging of surface structures, defects, and adsorbed ions in ambient electrolytes. In a previous thesis, it has been shown that the ds-AFM method with its unique resolution to operando conditions can be used to study polar/ferroelectric model electrocatalysts under operando conditions. Preliminary data and previous literature show indications for interesting effects when changing different parameters like, PH, salt concentration, local applied fields and applied potentials. [7, 8 ] However, additional research needs to be done to systematically study the effects of these external stimuli. Based on this enhanced understanding we will try to utilize these stimuli to tune our electrocatalysts while studying them in operando. Ds-AFM will be combined with x-ray photoemmision spectroscopy and kelvin force microscopy in respectively vacuum and air to compare surface charges in liquid, vacuum and air to further investigate the effect of the environment.
RESEARCH QUESTIONS
In this master thesis you will focus on answering the following questions:
· What is the local surface charge or potential of a polar/ferroelectric model system structure in
different atmospheres (vacuum, air, water). How does the history of the sample influence this?
Can we use the atmosphere to tune the surface charge?
F.e. change polarity with PH as shown in [8]
· How do the pH of the electrolyte, ions, and applied potential affect the local surface charge or
potential of a polar/ferroelectric model system structure change under applied potential?
And the morphology of the sample? And the local double layer environment?
· How do the morphology, surface charge and double layer affect OER activity measured
of the polar model catalysts?
· Possibly: How do the measured surface charges couple to polarization?
These questions will be addressed by using a multidisciplinary approach wit different characterization techniques as summarized in figure 1. Using atomic force microscopy operated in ‘dual-scale’ mode, which combines colloidal scale AFM spectroscopy with somewhat larger tips (radius 5–50 nm) with atomic resolution imaging using ultra-sharp tips (radius 1-2 nm), you will measure local surface potentials with a lateral resolution of ≤10nm in combination with atomic resolution imaging of surface structures, defects, and adsorbed ions in of ambient electrolytes of a range of PHs containing different ions. Kelvin probe microscopy (KPFM) will be used to measure the surface charge of the polar catalyst in air. X-ray photoemission spectroscopy (XPS) will be used to determine the accumulation of charges at the surface in vacuum. X-ray diffraction will be used to verify crystallinity of model systems. Moreover, films will be grown using pulsed laser deposition. All these equipment is readily available at the labs of Mesa+ and PCF.
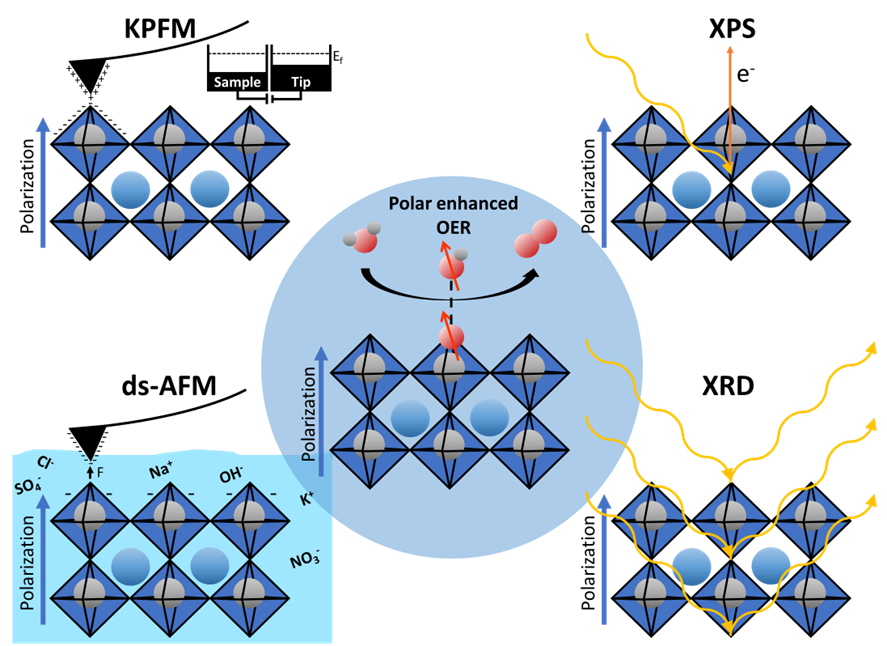
Figure 1. Summary of techniques which will be used in this master thesis.
LEARNING OBJECTIVES
In addition to the standard learning objectives for a Master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
· Learn how to work with Atomic Force Microscopy in air/liquids and Electrochemical AFM
(EC-AFM)
· Learn how to do pulsed laser deposition (PLD) and pre-characterize thin film catalysts using X-ray
diffraction (XRD) and X-ray Photoemmision spectroscopy
· Learn how to work with kelvin probe AFM
· Acquire or increase your lab experience with thin film catalysts
· Learn fundamental concepts of electrocatalysis
CONTACT INFORMATION
Dr. Igor Siretanu (daily advisor)
MSc. Emma van der Minne (daily advisor)
Prof. Frieder Mugele (thesis supervisor)
Dr. Chris Baeumer (thesis supervisor)
REFERENCES
[1] Y. Li, J. Li, W. Yang, and X. Wang, “Implementation of ferroelectric materials inphotocatalytic and photoelectrochemicalwater splitting,” Nanoscale Horiz, vol. 5, p. 1174, 2020, doi: 10.1039/d0nh00219d.
[2] A. Kakekhani and S. Ismail-Beigi, “Polarization-driven catalysis via ferroelectric oxide surfaces,” Phys. Chem. Chem. Phys, vol. 18, 1967, doi: 10.1039/c6cp03170f.
[3] A. Kakekhani, S. Ismail-Beigi, and E. I. Altman, “Ferroelectrics: A pathway to switchable surface chemistry and catalysis,” Surface Science, vol. 650, pp. 302–316, 2016, doi: 10.1016/j.susc.2015.10.055.
[4] A. Vijay, K. v. Ramanujachary, S. E. Lofland, and S. Vaidya, “Role of crystal structure and electrical polarization of an electrocatalyst in enhancing oxygen evolution performance: Bi-Fe-O system as a case study,” Electrochimica Acta, vol. 407, p. 139887, Mar. 2022, doi: 10.1016/J.ELECTACTA.2022.139887.
[5] I. Efe, N. A. Spaldin, and C. Gattinoni, “On the happiness of ferroelectric surfaces and its role in water dissociation: The example of bismuth ferrite,” Journal of Chemical Physics, vol. 154, no. 2, 2021, doi: 10.1063/5.0033897.
[6] A. Kakekhani and S. Ismail-Beigi, “Ferroelectric-Based Catalysis: Switchable Surface Chemistry,” ACS Catalysis, vol. 5, no. 8, pp. 4537–4545, 2015, doi: 10.1021/acscatal.5b00507.
[7] Master thesis Nynke Wijnant, Characterization of BiFeO3 as thin film polar model electrocatalyst, 2024, University of Twente
[8] Tian, Y., Wei, L., Zhang, Q. et al. Water printing of ferroelectric polarization. Nat Commun 9, 3809
