Background
The hydrogen evolution reaction (HER) and hydrogen oxidation reaction (HOR) are the foundation stones of the renewable hydrogen economy and are core to the transition towards a sustainable carbon-neutral economy. Electrocatalytic water splitting is arguably the most critical reaction for the up-coming H2 economy. A standing puzzle in the fundamental hydrogen evolution even on the model system electrocatalyst Pt (111) is the origin of the orders of magnitude decrease in reaction kinetics when moving from acid to alkaline, which has seriously retarded the further development of electrochemical energy technologies and therefore has attracted great recent research interest. So far, there are several theories to explain the kinetic sluggishness of HER/HOR in alkaline environments and/or in the presence of specific ions in the electrolyte, for example: i) the hydrogen binding energy (HBE) theory, which states that the variation in surface charge in the double layer attracts and traps (in the double layer) the adsorbed H as well as H+, increasing the hydrogen binding energy; ii) the proton donor theory; iii) the interfacial water reorganization theory, where the highly organized surface hydration layers and H-bond networks act as barriers for proton transport to/from the surface that severely inhibit the hydrogen electrocatalytic reactions; and so on1-3. However, these theoretical models are still under extensive debate, and little attention has been paid to the critical role of the surface charge, structure of the electric double layer (EDL), and interfacial hydration layers in electrocatalysis. So far, progress in understanding these processes has largely been hampered by the absence of suitable experimental techniques to characterize the electrocatalyst-electrolyte interface in situ and under operando conditions, as well as the quantification of local surface properties such as potentials, charge densities, defect distributions, and hydration effects with the required sub-particle or nanometer resolution. Recent improvements in various fields now allow for a detailed scrutiny of electrocatalytically active solid electrolyte interfaces from different experimental perspectives. In particular, we developed a dual-scale Atomic Force Microscopy (ds-AFM) method that allows the determination of local surface potentials with a lateral resolution of ≤10nm in combination with atomic resolution imaging of surface structures, defects, and adsorbed ions in ambient electrolytes. While earlier applied to insulating mineral particles, we recently used this technique for the first time to study photocatalytically active faceted nanoparticles of SrTiO3 and demonstrated that (i) different facets display opposite surface charges within a specific pH range of 4-6, (ii) hydration is very facet-dependent, and (iii) defect-rich regions along the boundaries of adjacent facets edges contribute significantly to the total charge of the NPs (Figure 1 c)4. In this master project, we want to extend the ds-AFM method with its unique resolution to operando conditions to faceted platinum electrocatalyst nanoparticles (Figure 1 b), such that we will be able to follow the response to changes in fluid composition and applied potential of the local (facets) surface properties mentioned above. We expect that the resulting physical insights will lead to optimized hydrogen evolution reactions at platinum electrocatalysts.
Research questions
· What structural features of platinum nanoparticles ranging from the micrometer to the atomic scale change during the electrochemical operation? How are the defects distributed on an electrode surface? And investigate whether the surface is static or dynamic, i.e., undergoes surface reconstructions during electro-chemical reactions. How is the active platinum electrode surface structure affected by variations in electrolyte composition (pH and concentration of specific ions)?
· What is the local surface charge or potential of platinum nanoparticle facets and molecular double-layer structure near a conducting Pt electrocatalyst electrode? How are the EDL and EDL forces evolving during reactions?
· What are the water molecule structure and interfacial hydration forces next to the passive and electrochemically active electrocatalyst surface? How do the pH of the solution, ions, and applied potential affect the hydration structure at the Pt-electrolyte interface?
· What is the optimal applied potential, the local double layer environment for effective hydrogen evolution reactions at platinum nanoparticles, and a sufficiently robust surface structure?
These questions will be addressed using atomic force microscopy operated in ‘dual-scale’ mode, which combines colloidal scale AFM spectroscopy with somewhat larger tips (radius 5–50 nm) with atomic resolution imaging using ultra-sharp tips (radius 1-2 nm). AFM measurements in fluid will be performed in a dynamic mode with small oscillation amplitudes (<1nm) to enable high lateral and temporal force resolution. Two AFM setups are available (Cypher ES from Asylum Research and ICON from Bruker). Both instruments are equipped with electrochemical liquid cells enabling fluid exchange and electrochemical potential control (bi-potentiostat). Samples will be prepared by controlled solid state dewetting5, 6 that is, the heat induced agglomeration of thin metal films into defined nanoparticles. Pt thin films (e.g., 5 nm-thick or so) will be deposited by magnetron sputtering onto electri5cally conductive (Nb-doped) SrTiO3 single crystal substrates. The samples will then be heat treated to convert the Pt film into a “monolayer” of spaced, faceted Pt NPs, with an average size of, e.g., 100-200 nm. Controlled faceting will be achieved by tuning the orientation of the single crystal substrate and the heat treatment conditions, e.g., temperature, duration, and gas.
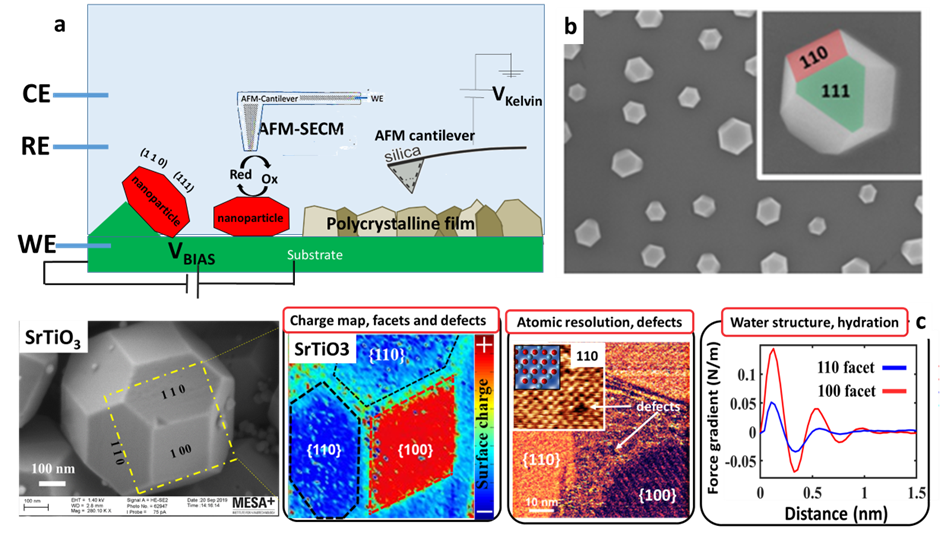
Figure 1. a) Experimental setup sketch for the measurements dynamic AFM in Electrochemical cell. b) SEM image of dewetted, faceted Pt NPs formed on a SrTiO3 single crystal substrate (unpublished data from Harsha et al.7 c) SEM image on SrTiO3 nanocrystal; 2D charge map across (100) and (110) facets (red: positive charge, blue:-negative charge); Oscillatory hydration forces show that water molecules are more ordered on (110) facet (higher amplitude) High-resolution phase image of corner between several facets displaying steps and domains of crystalline and disordered structure. Inset: atomic resolution topography images on (100) facet in liquid. It displays a vacancy defect and square lattice in agreement X-ray resolved structure. (data from Su et. al.4)
Learning objectives
In addition to the standard learning objectives for a Master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
· Learn how to work with Atomic Force Microscopy in air/liquids and Electrochemical AFM (EC-AFM)
· Acquire or increase your lab experience with faceted nanoparticle electrocatalysts
· Learn fundamental concepts of electrocatalysis
Contact information
Dr. Igor Siretanu (daily advisor) (i.siretanu@utwente.nl)
Dr. Marco Altomare (daily advisor) (m.altomare@utwente.nl)
Prof. Frieder Mugele (thesis supervisor) (f.mugele@utwente.nl)
References
(1) Li P; Jiang Y; Hu Y; Men Y; Liu Y; Cai W; Chen S. Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nature Catalysis 2022, 5 (10), 900-11
(2) Wilson, J. C.; Caratzoulas, S.; Vlachos, D. G.; Yan, Y. Insights into solvent and surface charge effects on Volmer step kinetics on Pt (111). Nature Communications 2023, 14 (1), 2384.
(3) Ledezma-Yanez, I.; Wallace, W. D. Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J. M.; Koper, M. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nature Energy 2017, 2 (4), 1-7.
(4) Su, S.; Siretanu, I.; van den Ende, D.; Mei, B.; Mul, G.; Mugele, F. Facet‐dependent surface charge and hydration of semiconducting nanoparticles at variable pH. Advanced materials 2021, 33 (52), 2106229.
(5) Thompson, C. V. Solid-state dewetting of thin films. Annual Review of Materials Research 2012, 42, 399-434.
(6) Altomare, M.; Nguyen, N. T.; Schmuki, P. Templated dewetting: designing entirely self-organized platforms for photocatalysis. Chemical science 2016, 7 (12), 6865-6886.
(7) Harsha, S., Sharma, R.K., Altomare, M., in preparation.
