Introduction
Due to a sustained progress in making and characterizing colloids over decades, many composite colloids are currently being developed. The potential application scope is broad, including the mimicking of individual molecules, controlling the collective optical and mechanical properties of suspensions, enhancing catalytic performance and more. The design principles vary per system, and same for the amount of complex colloids that can be obtained. One synthesis pathway that is currently under-explored is colloidal self-assembly via electrostatic heteroaggregation. Using oppositely charged ‘host’ and ‘guest’ particles, a variety of complex colloids can in principle be made. This principle has already been demonstrated in our group for spherical raspberries and faceted photocatalytists (see Fig)
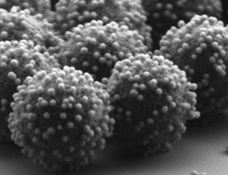
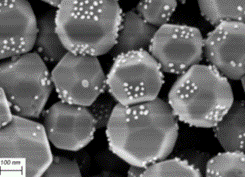
Examples of recent work where electrostatic interactions were used to assemble complex colloids. Left: all-silica raspberry colloids. Right: SrTiO3 particles with a facet-selective coating of Au nanoparticles.
Research Objectives
We will take self-assembly to the next level by using mixtures of guest particles. The latter will carry the same (positive/negative) type of charge but the size and/or magnitude of the (zeta) potential will be different. Key questions are: i) How will the two types of guests compete with each other for adsorbing onto the hosts? and ii) What are the roles of kinetics and thermodynamics in the self-assembly process?
The guest particles will repel each other if they carry similar charges. Even so, it is not clear to what extent kinetics or thermodynamic equilibrium will determine the final outcome. Small guest particles diffuse faster but may be less strongly attracted to the host particle. Reversible adsorption by both guest types should lead to an equilibrium occupancy of the host particle surface, but not necessarily under all ambient conditions. Even in case of irreversible adsorption, the adsorbed guest particles might have freedom to ‘roam’ the surface of the host, and thereby form a (quasi) 2D-equilibrium structure.
Experiments will be performed with confocal fluorescence microscopy and supported with other methods like SEM. Use of large host particles, and guest particles labeled with different fluorecent dyes, should allow distinguishing all three types of particles in live assembly experiments. Initially the host particles will be attached to a glass slide. The competition between the small and large guests will be influenced via their concentrations and via the pH and salinity of the liquid. For self-assembly in the bulk liquid, the overall concentration of the guests has to be high enough to ensure colloidal stability of the assembly. When studying the dynamics in concentrated systems, adsorbed and non-adsorbed guest particles have to be distinguished via the magnitude of their Brownian motion. This could be achieved by tracking the motions of the particles.
Learning Objectives
In addition to the standard learning objectives for a Master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will learn how to:
· prepare colloidal suspensions in a chemical lab (with or without sytnhesis)
· perform CSLM experiments
· extract quantitative information from the image-time series
· use insights from colloid science to interpret your findings
Contact Information
· Daily Supervision: Dr. Michel Duits (m.h.g.duits@utwente.nl)
· Supervision: Prof. Dr. Frieder Mugele (f.mugele@utwente.nl)
