Overview
Calcium carbonate (CaCO3) is one of the most widespread minerals on earth. Thanks to its rather high solubility in water, it plays a very important role in the global carbon cycle in both inorganic geological as well as biological mineralization and CO2 fixation processes. Exposure to water can lead to dissolution as well as precipitation of various forms of amorphous as well as crystalline CaCO3 as well as transformations between different crystal structures (polymorphs). Recently, researchers at PCF observed that surfaces of calcite, the most stable polymorph of CaCO3 under ambient conditions, undergo a rather peculiar transformation upon exposure to salty water. As shown in the figure below, initially atomically smooth cleavage surfaces of calcite break up into an array of individual domains with characteristic dimensions of a few hundred nanometers. Each of these domains seems to be atomically smooth at its surface with its edges align with the crystallographic axes of the underlying calcite crystal (see figure).
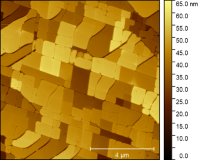
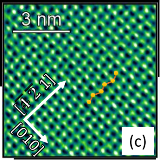
Left: Atomic Force Microscopy image of a {0104} cleavage surface of calcite after exposure to artificial sea water (a combination of NaCl, KCl, CaCl2, MgCl2) for approx. one week. Right: atomically resolved image of the surface lattice upon zooming into onto a homogeneous terrace.
Research Objective
The big question of this Master assignment is to identify the physical mechanism that is responsible for the formation of these domains. To this end, you will perform Atomic Force Microscopy measurements in ambient aqueous electrolytes of variable composition (salt content, pH) to follow the evolution from an atomically flat cleavage surface to the pattern shown observed. How does this transition depend on the type of anions and cations and on their concentration in the fluid? Is this transformation accompanied by extensive dissolution and re-crystallization of CaCO3? Does it involve the incorporation of other ions into the lattice that generate elastic stresses? Does the transformation entail changes in the local surface charge that might affect the chemical reactivity?
Learning Objective
In addition to the standard learning objectives for a master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
- Learn to perform and interpret high resolution in situ Atomic Force Microscopy experiments
- Learn about the physical chemistry of aqueous electrolyte and solid-electrolyte interfaces (which are both ubiquitous in nature and technology)
- Have basic chemical-lab training (preparing solutions and surfaces, etc.)
Contact Information
Daily Supervision: Dr. Igor Siretanu (i.siretanu@utwente.nl)
Supervision: Prof. Dr. Frieder Mugele (f.mugele@utwente.nl)
