Overview
Direct semiconductor-based photocatalytic conversion of solar energy to chemical fuels is considered an ideal renewable energy resource for the future. Yet, the relatively low energy conversion efficiency of current materials and systems has been limiting practical applications up until now. According to the current paradigm, optimum performance is achieved by using faceted semiconductor nanoparticles and by functionalizing them in a facet-selective manner with suitable cocatalysts for the desired redox reactions, such as water splitting or CO2 reduction. In literature, it is often shown that Ag, Au, and Pt occur preferentially on electron-accumulating facets ({010} for BiVO4), whereas the oxidative deposition of MnOx and PbO2 takes place on hole-accumulating facets ({110} for BiVO4) (Fig. 1a-b). Recently, researchers at PCF observed, using the specific example of BiOBr and BiVO4 particles, photo-induced reductive or oxidative deposition of co-catalysts onto plate-like semiconductor particles is pH-dependent and strongly affected by the presence of alkali chloride salts (Fig. 1c-d).
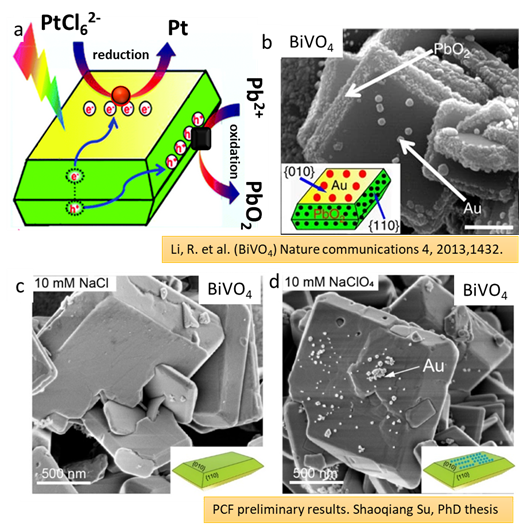
Figure 1. Sketch of selective photo-deposition of cocatalysts on BiVO4. SEM image of dual components (Au/PbO2/BiVO4) photo-deposited on the surface of BiVO4. Anion effects on the reductive and oxidative photo deposition (c-d). Reductive photo deposition of Au in 10 mM NaCl (b), 10 mM NaClO4 (d).
Research Objective
The big question of this Bachelor assignment is to identify how fluid composition (pH and salt content) influence cocatalyst deposition (metal/metal oxide) on faceted nanoparticles. To this end, you will perform the photodeposition of cocatalyst nanoparticles on well-defined, faceted BiVO4 (or SrTiO3) and quantify the influence of fluid composition. The morphologies and microstructures of the as-prepared samples will be analyzed by high-resolution Scanning Electron Microscopy. From these measurements, you will extract the location (facets and defects) and size of the cocatalyst, their coverage, as well as the amount of deposited cocatalyst. Depending on your expertise and/or interest, you can quantify the photocatalytic activity of materials using gas chromatography technology or assessed by dye degradation from the PhotoCatalyticSystems group. Controlled deposition of cocatalysts (metal or metal oxide) on faceted nanoparticles via fluid composition is easier as compared to controlling particle synthesis protocols. This could potentially give rise to better photocatalytic performance and stability for the materials.
Learning Objective
In addition to the standard learning objectives for a Bachelor’s/Master’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
- Obtain knowledge on photocatalysis; NPs colloidal interaction; ions adsorption
- Learn about the physical chemistry of aqueous electrolyte and solid-electrolyte
interfaces (which are both ubiquitous in nature and technology) - Have basic chemical-lab training (preparing solutions and surfaces, etc.)
- Learn how to handle colloidal suspensions and carry out photo-deposition
of cocatalyst - Depending on your interests, may analyze the samples activity using Gas
Chromatography
Contact Information
Daily Supervision: Dr. Igor Siretanu
Daily Supervision: Dr. Michel Duits
Supervision: Prof. Dr. Frieder Mugele
