Directly Probing the Effects of Anions on Hydration Forces
Overview
The interfacial water structure and the associated short-range hydration forces have long been recognized as essential for many phenomena and processes in nature and technology, including the stability of colloidal systems, the assembly of soft biological and non-biological matter on molecular and supramolecular scales, wetting, water desalination, lubrication, and catalysis, including in particular electro-(phooto)catalytic water splitting. In recent years, advances in atomic force microscopy technology have enabled the imaging and probing of Derjaguin–Landau–Verwey–Overbeek (DLVO) and hydration forces at solid-liquid interfaces with unprecedented resolution (Fig. 1a.). The charge and polarizability of the interface and surrounding ions, ion valency, and concentration are all expected to play a role in determining the relative magnitude of hydration forces. Recently, researchers at PCF observed that hydration forces (oscillatory and a monotonically decaying part) between sharp silica AFM tips at mica-water interfaces are strongly affected by the presence of alkali chloride salts (Fig. 1b.). The monotonic hydration force gradually decreases in strength with decreasing bulk hydration energy, leading to a transition from an overall repulsive (Li+, Na+) to an attractive (Rb+, Cs+) force. The oscillatory part, in contrast, is hardly affected by the presence of strongly hydrated cations (Li+, Na+), but it becomes completely suppressed in the presence of weakly hydrated cations (Rb+, Cs+) (Fig. 1b.).
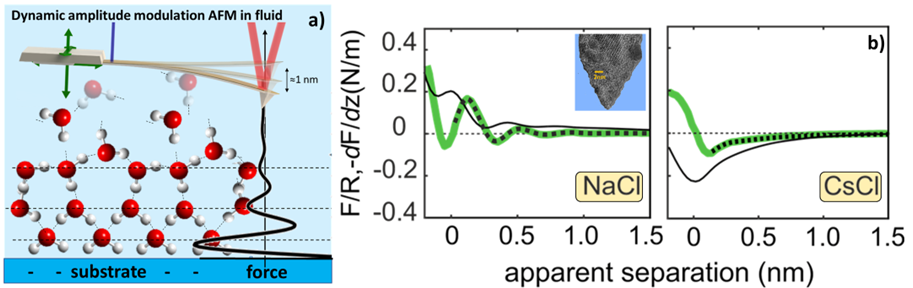
Figure 1. a) Schematic of water layers with highly ordered surface-bound layers of opposite polarity and gradually increasing positional and orientational order with increasing distance from the solid surface. Schematics for the AFM measurement on hydration layers on a negatively charged surface. b) Averaged force gradient (−dF/dz; thick green lines) and normalised force (F/R; thin black lines) versus apparent tip-sample separation measured in 50 mM NaCl and CsCl solutions..
Research Objective
The big question of this Bachelor assignment is to identify how anions influence hydration forces on mica surfaces. To this end, you will perform Atomic Force Microscopy measurements in ambient aqueous electrolytes of variable composition (salt content, pH) to follow the evolution of hydration forces for a set of common anions (ClO4−, NO3−, and SO42−) with Na+ and Cs+ as the co-ions. It will also be explored how the hydration forces depend on the AFM tip type and surface charge.
Learning Objective
In addition to the standard learning objectives for a Bachelor’s project (research planning, academic writing, data presenting, how to work in a lab environment, etc.), you will:
· Learn to perform and interpret high resolution in situ Atomic Force Microscopy experiments
· Learn about the physical chemistry of aqueous electrolyte and solid-electrolyte interfaces (which are both ubiquitous in nature and technology)
· Have basic chemical-lab training (preparing solutions and surfaces, etc.)
Contact Information
Daily Supervision: Dr. Igor Siretanu
Supervision: Prof. Dr. Frieder Mugele
