Can narrow spaces between dissolving olivine grains induce formation of new mineral layers?
Olivine is a silicate mineral whose dissolution produces Mg ions that can react with CO2 and store it permanently as MgCO3 (Negative Emission Technology). However the dissolution [1] can drastically slow down due to the formation of surface alteration layers (SALs) that hinder ion transport from the mineral to the electrolyte. Currently, the PCF group is studying mm-scale olivine grains that dissolve in a salt solution at low pH, thereby releasing Mg, Fe, and SiO4 ions. In a cm-scale liquid container, this surface reaction leads to strong concentration gradients because the ion diffusion is too slow to homogenize the liquid. As a consequence, new solid phases may become locally supersaturated and precipitate [2]. These locations are easy to predict for an isolated grain in a large container, but if the grain gets close to another grain or a container wall, the spatio-temporal concentration profiles get richer (see snapshot in Figure A).
Research objective
The goal of the BSc project is to explore the occurrence of local solid precipitation, where the preferred locations and expected timescale can be ‘engineered’ via i) the geometry of the grain(s) and the reactor, and ii) reaction conditions like pH and temperature. Numerical simulations of the reaction-diffusion problem will be helpful in the design of the experiments, which are performed with Confocal Raman Microscopy (CRM). With CRM we can detect surface retraction as well as chemical changes near the solid-liquid interface (local pH variation, mineral reprecipitation) (see Figure B, C and reference [3]).
Learning objectives
Through this project, you will gain (besides the common objectives for a BSc project) experience in modern in-situ experimental techniques, such as CRM, and in running simulations. A significant component involves data interpretation, including analysing CRM spectra, compositional maps, and thermodynamic aspects of solid precipitation.
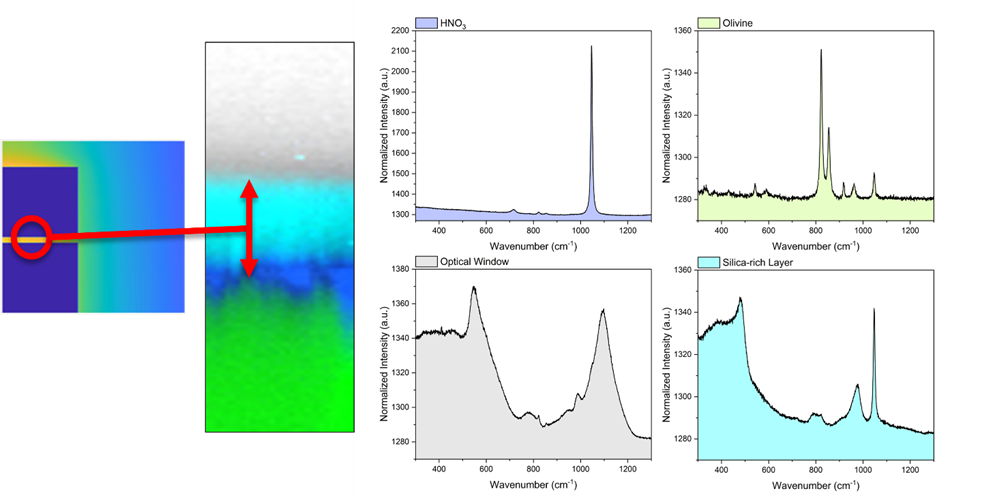
A: simulated Mg2+ concentration profile in a liquid cell with 2 dissolving olivine grains. B: Raman micro-scopy map, showing the formation of a silica layer in the narrow space between an olivine grain and a glass slide. C: Raman spectra of the 4 phases in the CRM map.
[1]: E.H. Oelkers et al., Chemical Geology 2018 (review on olivine dissolution)
[2]: H. King et al., Environmental Science & Technology 2010 (Si-rich SAL formation)
[3] T. Geisler et al., Nature Materials 2019 (SAL detection with CRM)
Daily guidance:
Vincenzo Alagia
Shilpa Mohanakumar
Supervision:
Frieder Mugele
