- Gerwin Steen - PhD student
- Herman Offerhaus - Chair
This project is a collaboration between Wetsus, centre of excellence for sustainable water technology and the Optical Sciences group.
Current methods of evaluation of ionic indicators are, for example, ion chromatography and ion selective membranes, however an alternative method which uses NIR light seems promising.
To investigate the possible use of near IR-light, multiple solutions of inorganic salts have been measured by near-IR spectroscopy, in a concentrations range of 0.5 to 0.03 M.
Measuring method
Overtones of water bands in near-infrared (NIR) spectroscopy provide information of the strength of vibration bonds. The influence of ions on these bonds can therefore be detected. To observe these effects, absorbance spectra are taken for both demi-water with and without certain concentration of an electrolyte.
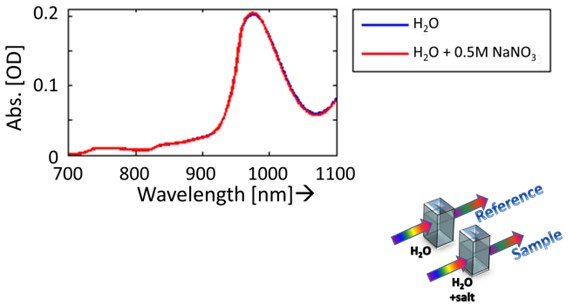
Fig 1. Absorbance spectrum of demi-water and a electrolyte (NaNO3).
The difference between measured absorbance spectra is small, as seen in figure 1. The absorbance spectrum of the reference (demi-water) is subtracted from the electrolyte absorbance spectrum to observe these small changes.
Difference spectra - NaNO3
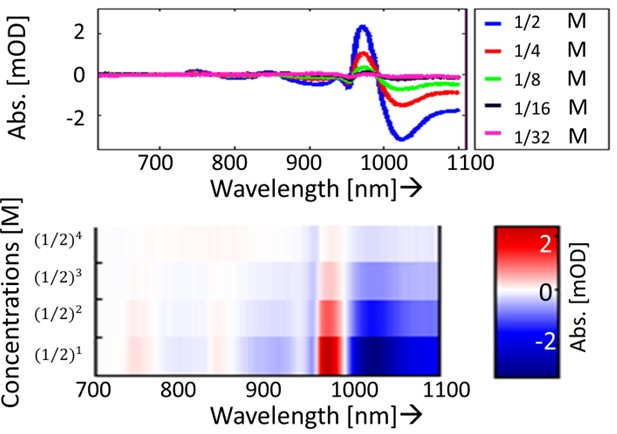
Fig 2. (top) Spectra of five different concentrations of NaNO3, (bottom) Different representation of the five different concentrations of NaNO3; the spectra are stacked on each other and their difference absorbance values are color indicated.
Fingerprinting electrolytes
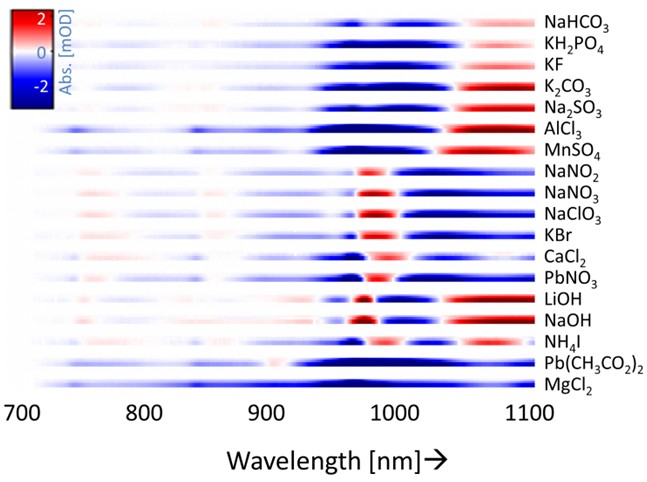
Fig 3. Fingerprinting
By measuring the change in absorbance of the third overtone of water bands, insight can be gained on the perturbations of ions on water. Distinguishable features can be observed for different electrolytes after subtracting the spectra of the solution from reference spectra of demi water.
The technology is to be integrated into high-tech but low-cost optofluidic chips which enable direct and fast concentration measurement of relevant ions in drinking water.
