Courses
The Membrane Surface Science group participates actively in the teaching of the Chemical Engineering curriculum, both in the BSc and the MSc phase. The following are the courses offered and their details:
- 193735000 - C.S. Membrane Technology
- 201200117 - Membranes for Gas Separation
- 201300049 – Advanced Molecular Separations
BSc and MSc projects
Many different BSc and MSc project are possible within the MSuS group. Projects can involve making or coating membranes (Material Science), but can also be more focused on characterizing membranes and optimizing their performance for a specific application (Process Technology). For a most up-to-date overview of possible projects please contact w.m.devos@utwente.nl.
Some examples of possible projects are given below:
- Effect of Nanoparticles Incorporation in Polyelectrolyte Multilayer Membranes
The accumulation of various pollutants such as organic micropollutants (OMP) and perfluoroalkyl substances (PFAS) in surface and groundwaters used for the production of drinking water poses a serious threat to environmental and human health. These compounds often enter the environment through our wastewater treatment facilities, which are currently ill-equipped to deal with such contaminants. Therefore, there is a need to improve our current water cleaning techniques. One of the promising techniques for cleaning such aqueous waste streams is asymmetric polyelectrolyte multilayer membranes (PEMM). These membranes have shown great promise due to their ability to still remove small components such as OMPs, while maintaining a high permeability towards water [1]. These asymmetric PEMMs achieve this by being able to deposit a thin nanometre-thick separation layer. They do this by using the Layer-by-Layer (LbL) technique, a method that involves the alternate deposition of oppositely charged polyelectrolytes on a porous ultrafiltration support membrane, to first deposit an “open” bottom polyelectrolyte pair that has a high permeability and low selectivity towards pollutants followed by a nanometre thick “dense” top layer that has a low permeability, but high selectivity. This open bottom layer causes the pores to fill up and form a uniform layer, that has a low selectivity, but high permeability. Due to the pores of the support membrane already being closed off by the bottom-layer only a very thin top-layer is necessary, causing the added resistance towards mass transport of water over the membrane to be relatively low resulting in high permeabilities. The selectivity of the membrane is however already fully determined by the top-layer resulting in high retentions of pollutants at high permeabilities.
Although the LbL technique is relatively simple, it involves multiple deposition steps to achieve the desired membrane structure and thickness, making the process time-consuming and labor-intensive. Each layer must be individually applied, often requiring many repetitions to build up a functional membrane. Incorporating nanoparticles into the LbL process can offer a way to reduce the number of steps, as nanoparticles are much larger than individual polyelectrolytes they can significantly increase the adsorption of material in a single deposition cycle. This higher material loading in each step could potentially not only accelerate the membrane assembly but also offer new interesting separation properties leading to PEMMs being utilized for new separations.
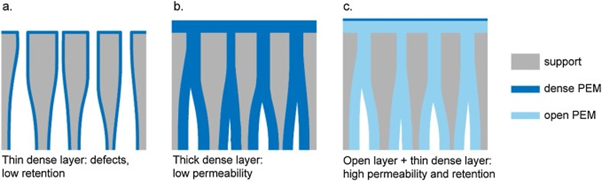
Figure 1: Concept of an asymmetric PEMM showing how a thin selective layer is achieved [1].
This project investigates the effect of incorporating nanoparticles into polyelectrolyte multilayers during the LbL deposition process. By incorporating nanoparticles at different stages of the LbL assembly, the student will explore the changes in the growth curves of the multilayer on wafer substrates with techniques like reflectometry or ellipsometry. Additionally, membrane and module fabrication as well as cross-flow experiments will be performed to investigate the membrane separation properties such as permeability, salt rejection, and molecular weight cut-off. The student will hereby investigate the relationship between different nanoparticles and coating conditions on the eventual membrane performance.
If you want to know more about this project, please contact Roberto Andrade and Tjerk Watt (j.r.andradeaguirre@utwente.nl, t.r.watt@utwente.nl)
References
[1] te Brinke, E., Reurink, D. M., Achterhuis, I., de Grooth, J., & de Vos, W. M. (2020). Asymmetric polyelectrolyte multilayer membranes with ultrathin separation layers for highly efficient micropollutant removal. Applied Materials Today, 18, 100471.
- Developing Dense Polyelectrolyte Multilayer Membranes for organic micropollutant removal
Organic micropollutant (OMP) contamination in water, such as pharmaceuticals, pesticides, and personal care products, is an emerging concern for global water treatment efforts. Although present in trace amounts, these OMPs can be harmful even at very low concentrations. Due to their small molecular sizes, many existing filtration technologies struggle to remove them from water sources effectively. As water scarcity intensifies due to population growth, industrial activities, and climate change, the need for advanced filtration technologies becomes crucial.
Polyelectrolyte Multilayer (PEM) membranes, constructed by alternating layers of positively and negatively charged polyelectrolytes, exhibit unique properties such as tunable pore size, surface charge, and permeability. Current PEM membranes are either highly selective for OMPs or exhibit strong stability, but achieving both simultaneously remains a challenge. This project aims to develop membranes that balance high selectivity and stability to improve overall performance. This project aims to develop dense and stable PEM-based nanofiltration (NF) membranes for surface water treatment by testing novel polyelectrolyte pairs. Additionally, the membranes will be evaluated for their stability and performance in harsh conditions, ensuring they maintain their selectivity and efficacy against micropollutants even after repeated use, leveraging their advanced properties to improve water purification efficiency.
Keywords: Polyelectrolyte multilayers, nanofiltration, dense membranes
During this project, you will focus on:
· Conducting an in-depth review of current literature on PEM membranes, focusing on their working mechanisms, properties, and efficacy in water treatment applications. This review will cover key topics such as the properties of different polyelectrolytes, their ion selectivity behavior, and the latest innovations in PEM membrane technology.
· Fabricate PEM membranes using the layer-by-layer technique, focusing on dense membrane formation to enhance treatment efficiency.
· Performing experiments to evaluate the permeability, salt retention, MP retention, and molecular weight cut-off (MWCO) of PEM membranes.
· Explore symmetric and asymmetric coating configurations for optimized membrane performance.
· Comparing the performance of dense PEM membranes with conventional NF membranes, focusing on improvements in selectivity.
This project is ideal for master's students seeking hands-on experience in advanced water treatment technologies.
For more information, please contact: Sina Rezaei (Sina.rezaei@utwente.nl)
- Understanding pH-dependent odd-even effects in polyelectrolyte multilayer membranes
Esra te Brinke (1), Wiebe de Vos (1)
(1)Membrane Surface Science (MSuS), Membrane Science and Technology Cluster
Project background
Polyelectrolyte multilayer (PEM) membranes, formed by alternate adsorption of polycation and polyanion nanolayers on a membrane support, are recently becoming more and more important in membrane applications. One intrinsic feature of PEM membranes is that they have different properties depending on the terminating layer, the so-called odd-even effect. Not only the polarity of the membrane surface is determined by the terminating layer, but typically also the total charge density and therefore the swelling of the layer [1]. Therefore, besides ion retention also water permeability of the membrane is influenced by this odd-even effect, as well as the molecular weight cut-off of neutral molecules. This effect is very well visible as a zig-zag pattern in membrane permeability as a function of the number of coated polyelectrolyte layers (figure 1). During a recent project we have observed that the odd-even effect of poly(allylamine hydrochloride) and poly(acrylic acid) can switch depending on coating pH, i.e. that the coating pH can determine which of the two polyelectrolytes will induce most swelling as the terminating layer. However, both at low and high pH the odd-even effect was exactly opposite to what we expected, so apparently our theoretical framework is not complete. For a better fundamental understanding of PEM membrane production and properties, we want to further investigate this.
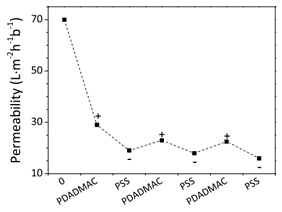
Figure 1. Odd-even effect on the water permeability when alternatingly coating poly(diallyldimethylammonium chloride) and poly(styrene sulfonate) layers [1].
Project description
The aim of this student project is to study an additional pH-sensitive polyelectrolyte pair and see if we can obtain similar results or not. Permeability and molecular weight cut-off will be determined by crossflow experiments and GPC. Additionally, we want to take a more fundamental look how pH affects the odd-even effect. PEM build-up will therefore be studied by reflectometry and ellipsometry. Hydrophobicity could be tested by contact angle measurements. Depending on the duration of the project, some of the experiments from the previous work might be repeated or additional measurements on that PEM system might be performed. Finally, we will try to relate fundamental insights into the performance of polyelectrolyte multilayer membranes produced with different terminating layers and coated under different pH conditions.
References
[1] J. de Grooth; A tale of two charges: Zwitterionic Polyelectrolyte Multilayer Membranes; doctoral thesis, Ipskamp printing Enschede, 2015
- Design of adaptive polyelectrolyte multilayer membranes
Esra te Brinke (1), Albert Wong(2), Wiebe de Vos(1)
(1)Membrane Surface Science (MSuS), Membrane Science and Technology Cluster
(2)Chemical Reaction Networks (CRN@UT), Department of Molecules and MaterialsContact: e.tebrinke@utwente.nl
Project background
Polyelectrolyte multilayer membranes are an emerging field in membrane technology because of their high versatility. To produce such membranes, a layer-by-layer coating of oppositely charged polyelectrolytes is applied on a porous membrane support (figure 1a-b). This leads to a relatively dense but thin separation layer with nanofiltration properties (molecular weight cutoff 200-1000 Da, high divalent salt retention). The exact properties can be controlled by various parameters such as layer thickness, choice of polyelectrolytes [1-2] and coating conditions [3]. Additionally, polyelectrolyte multilayers can be covalently crosslinked after the coating procedure to increase their density [1].
We wish to design membranes that have potential adaptive properties. In this master student project, you will make use of dynamic combinatorial chemistry to produce polyelectrolyte membranes that can crosslink, but also uncrosslink, upon specific chemical triggers. The objective is to create membranes of which the density and separation properties can be changed as a function of the chemical composition in the sample; the membrane can, thus, self-select its degree of separation. The formation of disulfide bonds by thiols is highly dynamic in nature and therefore very suitable for this purpose (figure 1c). Reversible tuning of membrane properties would be a first step towards new chemistries that allow for smart realtime control over membrane performance and the output of membrane processes.
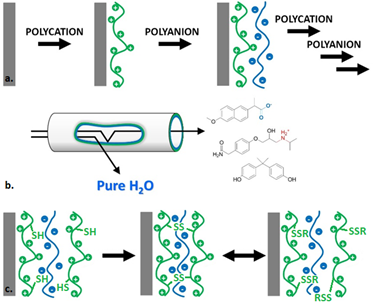
Figure 1. a. Polyelectrolyte multilayer coating; b. Polyelectrolyte multilayer membranes used as a tool to filtrate water (i.e. selectively separate unwanted chemical compounds such as pharmaceuticals from an aqueous sample); c. Reversible polyelectrolyte multilayer crosslinking via disulfide bonds.
Project description
The aim of this master student project is to synthesize a functionalized polylysine that can be used to coat polyelectrolyte multilayers and tune their properties by chemical triggers. The synthesis will be performed in the CRN group (lead by Dr. Albert Wong, https://www.utwente.nl/en/tnw/crn/), whereafter membranes will be produced and tested in the MSuS group (lead by Prof. Dr. Wiebe de Vos, https://www.utwente.nl/en/tnw/msus/). The project will involve many different experimental procedures common in physical organic chemistry (including synthesis, characterization, and kinetic assays), and in membrane science (including layer by layer coating, membrane module production, crossflow experiments and molecular weight cutoff determination by GPC). Additionally, techniques to determine multilayer buildup and properties such as reflectometry, ellipsometry and zeta potential measurements could be part of the project.
References
[1] D. M. Reurink, J. D. Willott, H. D. W. Roesink and W. M. de Vos; Role of Polycation and Cross-Linking in Polyelectrolyte Multilayer Membranes; ACS Applied Polymer Materials, 2020, 2:5278-5289
[2] L. Krasemann and B. Tieke; Selective Ion Transport across Self-Assembled Alternating Multilayers of Cationic and Anionic Polyelectrolytes; Langmuir, 2000, 16:287-290
[3] J. de Grooth, R. Oborný, J. Potreck, K. Nijmeijer, W. M. de Vos; The role of ionic strength and odd–even effects on the properties of polyelectrolyte multilayer nanofiltration membranes; Journal of Membrane Science, 2015, 475:311–319
- Crosslinking for further improvement of asymmetric polyelectrolyte multilayer membranes
Esra te Brinke (1), Wiebe de Vos (1)
(1)Membrane Surface Science (MSuS), Membrane Science and Technology Cluster
Project background
Membrane performance is not only determined by selectivity but also permeability, which determines the efficiency of the membrane. Permeability can be increased by decreasing the thickness of the selective layer. This idea led to the development of membranes that consist of a porous support for mechanical stability with a thin separation layer coated on top of the support. Layer-by-layer coating of oppositely charged polyelectrolytes, to form a polyelectrolyte multilayer (PEM) membrane, is one of the techniques that can be used for this purpose. However, in practice the coating will also partly fill the support pores which makes the separation layer still much thicker than necessary (figure 1). Recently, this problem was largely circumvented by coating asymmetric PEM membranes with a very permeable bottom layer (poly(styrene sulfonate) + poly(allylamine hydrochloride)) to close the pores, and a 4 nm thin, very dense PEM (poly(acrylic acid) + poly(allylamine hydrochloride)) on top of this as the separation layer [1]. This led to much higher water permeabilities compared with PEM membranes that are completely coated with the dense PEM, and 98% retention of organic micropollutants such as medicines and pesticides. However, further investigation showed that the bottom and top layer are mixed to some extent [2]. This probably leads to a decrease in top layer density, such that the separation properties of the top layer are not yet fully exploited in these asymmetric membranes.

Figure 1. Dense vs. asymmetric PEM coating on a porous support membrane.
Project description
The aim of this student project is to covalently crosslink the bottom PEM of the asymmetric membranes with glutaraldehyde before coating the top layer, to inhibit mixing of the bottom and top layer. Crosslinking, however, also has its drawbacks: it densifies the PEM such that overall permeability will decrease, and it reduces the charge of the bottom layer which can affect the properties of the top layer. Therefore, the glutaraldehyde concentration and the crosslinking time need to be finetuned. The effect of crosslinking on the coating process of the top layer will be monitored by reflectometry and ellipsometry. Membrane performance will be determined by crossflow experiments, in combination with analytical techniques such as conductivity measurements, HPLC, HPLC-MS and GPC. Depending on the duration of the project it is also possible to investigate different bottom layer types or different crosslinking agents, or to perform additional crosslinking of the top layer to make it even thinner and denser.
References
[1] E. te Brinke, D. M. Reurink, I. Achterhuis, J. de Grooth and W. M. de Vos; Asymmetric polyelectrolyte multilayer membranes with ultrathin separation layers for highly efficient micropollutant removal; Applied Materials Today, 2020, 18:100471
[2] I. J. Gresham, D. M. Reurink, S. W. Prescott, A. R. J. Nelson, W. M. de Vos and J. D. Willott; Structure and Hydration of Asymmetric Polyelectrolyte Multilayers as Studied by Neutron Reflectometry: Connecting Multilayer Structure to Superior Membrane Performance; Macromolecules, 2020, 53: 10644-54
- Polyelectrolyte based gas barrier coatings for flexible packaging
Jiaying Li (1), Wiebe M. de Vos (1)
(1) Membrane Surface Science (MSuS)
Project background
Solvent-borne coatings with a relatively high content of Volatile Organic Compounds (VOCs) have traditionally dominated the coating industry, however, there are increasing health and safety concerns. A transition from solvent-borne coatings to more environmentally friendly coatings has been realized for consumer coatings in the late 1990s.1 Waterborne coating is one of the solutions and has gained enormous research attention in the modern coating industry. Key components of a waterborne coating are water, co-solvent, polymer binder, pigment, and various additives.2 Among all these ingredients, the primary properties of the coating film are determined by the binder.
To explore new possibilities, a new film formation system based on polyelectrolyte (PE) complexation is investigated. PEs are polymers with charged functional groups and usually have good water solubility. With a pH and salt control, the direct complexation is prevented and a homogenous solution mixture can be obtained. The mixture can then be cast onto a substrate with controlled thickness. Upon drying, complexation may occur due to a change in pH by an aqueous bath or evaporation. The possible routes are shown in Figure 1. A strong ionic network among PEs can be formed. Barrier properties and sufficient mechanical strength are expected to be achieved with cross-linking.
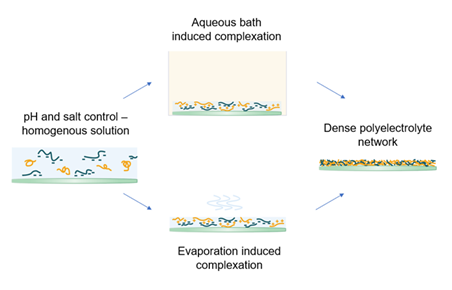
Figure 1: Two different routes of complexation
Project details and outcome
In this master project, the main goal is to study different ratios of polycation: polyanion as gas barrier materials. You will start to prepare high concentration of polycation: polyanion solutions in different ratios to understand how that influences the complexation. Different ratios lead to different degrees of ionic cross linking that define the final gas barrier performance. To better understand the microstructure, you will study the intrinsic complex properties by several techniques such as DSC and ellipsometry. During the film preparation, you will also get hands-on experience of different film characterization techniques, for example, SEM and AFM. Moreover you will learn how to perform gas permeation tests. Throughout the whole project, you will learn about water-based coating, polyelectrolyte complexation and gas permeation. Meanwhile, a link between academic research and industrial application is also made, as this project is in collaboration with BASF and AkzoNobel.
In this project, your main tasks are:
1. Preparation of PE solutions
2. Preparation of PE films on substrates
3. Characterizations of the PE films
4. Gas permeation measurements of the PE films
For more details and questions, please contact J. (Lily )Li, j.li-3@utwente.nl.
[1] K. D. Weiss, Paint and coatings: A mature industry in transition. Progress in Polymer Science 22, 203-245 (1997).
[2] E. Mehravar, J. Leswin, B. Reck, J. R. Leiza, J. M. Asua, Waterborne paints containing nano-sized crystalline domains formed by comb-like polymers. Progress in Organic Coatings 106, 11-19 (2017).
- Membranes by Aqueous Phase Separation (APS): Effect of polyelectrolyte molecular weights on the membrane properties
Muhammad Irshad Baig(1), Wiebe M. de Vos(1)
(1)Membrane Surface Science, Membrane Science and Technology Cluster, MESA+ Institute for Nanotechnology, University of Twente, Faculty of Science and Technology, P.O. Box 217, 7500 AE, Enschede, The Netherlands.
m.i.baig@utwente.nl ; w.m.devos@utwente.nl
Project Background
A membrane is a barrier that regulates the flow of species in a selective way. The most commonly used membranes in water purification and gas separation applications are made from polymers that are only soluble in organic solvents. Ask anyone who works in the field of polymeric membranes about its fabrication procedure, and you will mostly hear one answer i.e. NIPS. It stands for Non-solvent Induced Phase Separation. Simply put, you take a polymer (Polysulfone, Polyimides for example) and dissolve it in an organic solvent such as N-methyl-2-pyrrolidone (NMP). When the solution is homogeneously mixed, it is then cast on a flat surface and put in a water bath. The solvent (NMP) is highly miscible in water and so it diffuses out of the polymer solution and mixes with water. The polymer, at this point becomes insoluble due to all the water that is around it, forming a solid porous membrane. The membranes prepared in this way are also known as ‘Loeb-Sourirajan Membranes (L-S membranes)’, named after the researchers who first made asymmetric cellulose acetate membranes for sea water desalination.

Figure 1. Schematic illustration of the polyelectrolyte complex membrane formation.
This simple procedure of making commercial polymer membranes has remained the same since the 1970’s. NMP is by far the most widely used solvent to prepare membranes and the multi-billion dollars membrane industry uses this solvent in immense amounts.
However, NMP is not a friendly chemical by any means. It is partly flammable, and most importantly, it has been proven to be repro-toxic to humans and harmful for the environment.1 Therefore, it has to be recycled and/or removed from waste streams plus it has to be removed from the membranes before they can be utilized for water production. The EU has introduced a law that restricts the use of such chemicals throughout the Union.2
aving discussed the nature of aprotic, polar organic solvents and their harmful effects, we have an alternative solution which uses only water as a solvent. It is extremely difficult and mostly impossible to dissolve conventional polymers in water. This leaves us with a special class of polymers called ‘Polyelectrolytes’ which can be easily dissolved in water. These polyelectrolytes are charged polymers with a unique property that their charge can be manipulated by a pH switch. Some polyelectrolytes are only charged below a particular pH. They are called weak polyelectrolytes. On the other hand, certain polyelectrolytes remain charged in almost all the pH range. These are called strong polyelectrolytes. When the two oppositely charged polyelectrolytes come across each other, they instantly form a ‘Polyelectrolyte Complex’. So if we make a homogeneous solution of these two polyelectrolytes such that they are uncharged in the solution and then cast it in a water bath with a pH where both become charged, we get a polyelectrolyte complex membrane (Figure 1). This novel approach to making membranes is termed ‘Aqueous Phase Separation’ (APS).
Project Details and Outcome
Project Details and Outcome
In this project, Poly(sodium 4-styrenesulfonate) and Polyethyleneimine will be used the polyelectrolytes, respectively. The aim of this project is to develop free-standing membranes using the two polyelectrolytes mentioned above. During the course of the project, Monomer mixing ratio, effect of coagulation bath pH, cross-linking conditions, and type of salt in coagulation bath would be investigated in detail.
The membranes prepared in this study will be used for water treatment and purification applications. The applications are as diverse as the new APS field itself. From oil/water separation (Microfiltration) to sea water desalination (nanofiltration and reverse osmosis), the outcomes of this study are diverse. It is noteworthy that one of the inventors of L-S membranes, Loeb Sidney, was a MSc. student at UCLA when he got the breakthrough in membrane science and technology. Similarly, this project, part of a larger research goal, will play an important role in shaping the next generation of polyelectrolyte membranes.
References
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551.
- The European Commission, Off. J. Eur. Union, 2018, 99, 3
- Influence of Ion Concentration on Polyelectrolyte Multilayer based Nanofiltration Membrane Performance
Influence of Ion Concentration on Polyelectrolyte Multilayer based Nanofiltration Membrane Performance (MSc assignment)
Introduction
In recent years a variety of micropollutants have been detected in ground- and surface water [1]. Micropollutants are small organic molecules with variable chemical properties that originate among others from industrial, medical and agricultural waste. Many of these molecules are highly toxic, carcinogenic or endocrine-disrupting compounds [2]. Even though the observed concentrations are still below drinking water guidelines, these micropollutants are potentially harmful to humans, organisms and the environment, as there is very little knowledge on longtime exposure and possible synergetic effects [3]. Traditional water treatment methods are not able to sufficiently remove these, therefore advanced separation technologies need to be developed to prevent them from accumulating in our water cycle [4].
Dense membranes used in pressure-driven filtration processes such as reverse osmosis (RO) or nanofiltration (NF) are promising techniques that have been shown to retain most micropollutants [5]. The advantage of nanofiltration membranes over reverse osmosis membranes is the reduced energy cost due to lower pressures at very comparable separation performances. A relatively young and promising method to make nanofiltration membranes is to coat a very thin and selective separation layer on top of an open porous support structure using the Layer-by-Layer (LBL) method, developed by Decher in 1997 [6]. In this method polyelectrolytes of different charged are alternately coated on top of a charged substrate. The layer formation is driven by electrostatic interactions between the polyelectrolyte chains and the entropic gain of counterion release.
Project details and outcome
In the cluster of Membrane Science and Technology these so-called Polyelectrolyte Multilayer (PEM) membranes are developed and investigated. Depending on the membrane coating conditions the structure and with that the membrane performance, solute selectivity and solvent permeability can be changed. At the same time, it is hypothesized, that the membrane performance directly depends on the type and concentration of ions present during filtration. In addition to ion adsorption and charge screening effects, commonly observed phenomena for nanofiltration, the PEM structure might change significantly for different ions and ion concentrations, which has been recently observed in QCM-D studies of PEM swelling behavior [7].
The aim of this research is to investigate the influence of ion concentration on PEM performance related to structural changes. The focus will be on macroscopic transport measurements conducted with coated ultrafiltration membranes. Simultaneously structural characteristics of the multilayer, coated on a model surface, will be investigated. Following these detailed experimental studies, the applicability of a nanofiltration model based on the extended Nernst-Planck equation for the prediction of membrane retention accounting for ion adsorption, charge screening and structural changes shall be investigated.
Your tasks:
· prepare and characterize PEM hollow fiber membranes
· conduct macroscopic transport measurements
· investigate swelling properties for different salts and salt concentrations
· apply a transport model to describe membrane performance
For more information please contact Moritz Junker (m.a.junker@utwente.nl)
1.Aa, N. G. F. M. v. d.; Dijkman, E.; Bijlsma, L.; Emke, E.; Ven, B. M. v. d.; Nuijs, A. L. N. v.; Voogt, P. d., Drugs of Abuse and Tranquilizers in Dutch Surface Waters, Drinking Water and Wastewater - Results of Screening Monitoring 2009. National Institute for Public Health and the Environment 2010.
2.Trapido, M.; Epold, I.; Bolobajev, J.; Dulova, N., Emerging micropollutants in water/wastewater: growing demand on removal technologies. Environmental science and pollution research international 2014, 21 (21), 12217–12222.
3.Verliefde, A.; Cornelissen, E.; Amy, G.; van der Bruggen, B.; van Dijk, H., Priority organic micropollutants in water sources in Flanders and the Netherlands and assessment of removal possibilities with nanofiltration. Environmental pollution (Barking, Essex : 1987) 2007, 146 (1), 281–289.
4.Tröger, R.; Klöckner, P.; Ahrens, L.; Wiberg, K., Micropollutants in drinking water from source to tap - Method development and application of a multiresidue screening method. Science of The Total Environment 2018, 627, 1404–1432.
5.Yangali-Quintanilla, V.; Maeng, S. K.; Fujioka, T.; Kennedy, M.; Amy, G., Proposing nanofiltration as acceptable barrier for organic contaminants in water reuse. Journal of Membrane Science 2010, 362 (1), 334-345.
6.Decher, G., Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277 (5330), 1232–1237.
7.O’Neal, J. T.; Dai, E. Y.; Zhang, Y.; Clark, K. B.; Wilcox, K. G.; George, I. M.; Ramasamy, N. E.; Enriquez, D.; Batys, P.; Sammalkorpi, M.; Lutkenhaus, J. L., QCM-D Investigation of Swelling Behavior of Layer-by-Layer Thin Films upon Exposure to Monovalent Ions. Langmuir 2018, 34 (3), 999-1009.

