Optical coherence tomography and high-fidelity flow simulations for improved stent planning in femoropopliteal disease
PROJECT ACRONYM
Optimo
FUNDED BY
Connecting industries call (Topsector HTSM, University of Twente, Abbott Vascular)
PHD CANDIDATE
Lisa Rutten
SUPERVISORS
Prof. dr. M.M.P.J. Reijnen
Prof. dr. M. Versluis
Co-supervisor:
Dr. K. Jain
COLLABORATION
Physics of Fluids group
Engineering Fluid Dynamics group
Abbott Vascular
Rijnstate
CLINICAL BACKGROUND
In up to 20% of the patients diagnosed with femoropopliteal disease and treated with a stent, an in-stent restenosis occurs within one year after treatment.[1,2] A subsequent reintervention is often needed. This project focuses on improving the treatment of patients diagnosed with femoropopliteal disease by improving the treatment planning and using computational fluid dynamics (CFD) models.
In current clinical practice, the treatment planning of femoropopliteal disease is typically based on a single-plane digital subtraction angiography (DSA). As DSA only provides a 2D image of a commonly tortuous lesion, vessel diameters are hard to define and small atherosclerotic plaques challenging to identify, particularly when located in the same plane as the DSA. Vascular lesions can also be visualized using optical coherence tomography (OCT), a catheter-mounted imaging technique that uses near-infrared light to obtain a 3D scan of the vessel lumen and vessel wall. To date, OCT is mainly used in interventional cardiology. For both pre- and post percutaneous coronary intervention OCT imaging influenced the DSA-based physician decision-making in 57% and 27%, respectively.[3] Furthermore, OCT-guided percutaneous coronary intervention was associated with improved procedural outcomes, less in-hospital events and better long term survival compared to standard DSA-guided percutaneous coronary intervention.[4] This, thus, indicates that OCT provides additional and important information on vessel characteristics that influence the treatment planning. Using OCT in the femoropopliteal tract was found to be safe, but the added value of OCT imaging on the decision-making process of endovascular treatment of femoropopliteal disease is not yet investigated.[5]
Another approach to improve the treatment of patients diagnosed with femoropopliteal disease is by predicting which patients do or do not develop an in-stent restenosis and using this information to personalize the treatment and follow-up. It was already shown that the formation of a stenosis is related to flow abnormalities, but current imaging techniques are unable or not always available to visualize the complex blood flow.[6] Complex blood flow can also be simulated using CFD models. Advantages of CFD are that non-measurable parameters, for example the wall shear stress, can be calculated and that the effect of a treatment, such as stent placement, on the blood flow can be investigated. This could transfer the general treatment to a personalized treatment. However, to obtain a reliable CFD model, adequate patient data is necessary, but not always available. Assumptions in the CFD model are, therefore, unavoidable and could affect the accuracy of the CFD model. Uncertainties in the CFD model could arise from the geometry, inlet-, outlet- and wall boundary condition and blood rheology. Investigating the effect of uncertainties in the input parameters on the CFD solution is necessary to obtain clinically feasible CFD models.
OBJECTIVE
Improving the endovascular treatment of patients diagnosed with femoropopliteal disease by:
- Investigating the added value of using OCT in the treatment planning of femoropopliteal disease
- Investigating the effect of uncertainties in input parameters on the CFD solution
- Improving the CFD models using OCT
ONGOING PROJECTS
Velocity-based inlet boundary condition
In this project the velocity-based inlet boundary condition obtained from duplex ultrasound (DUS) and ultrasound particle image velocimetry (echoPIV) will be compared. Since DUS is a 1D velocity measurement and echoPIV a 2D velocity measurement, we expect that using echoPIV to base the inlet boundary condition on, will result in a more patient-specific CFD model. Due to differences in the obtained velocity profile and flow rate, this results in the following simulations investigating the effect of the flow rate and velocity profile on the CFD solution:
- Womersley velocity profile, DUS-based flow rate (WOM-DUS)
- EchoPIV velocity profile, echoPIV-based flow rate (PIV-PIV)
- Womersley velocity profile, echoPIV-based flow rate (WOM-PIV)
Blooming artefact
CT angiography (CTA) scans are limited by blooming artefacts, causing high-density objects (for example stent struts) to appear larger than they actually are. As a result, the vessel lumen appears smaller than it actually is, thereby inducing uncertainty in the obtained geometry. In this project we focus on quantifying as well as reducing the blooming artefact. A possible solution to reduce the blooming artefact is to use high-energy virtual monoenergetic image reconstructions obtained from a detector-based spectral CT scanner.
(a) |
(b) |
Figure 1: Proximal stent marker causing a blooming artefact in a CTA scan. (b) Corresponding segmentation showing a stenosis that is not really present in the patient.
OCT-based treatment planning in femoropopliteal disease
In this project the DSA-based treatment planning will be compared to the OCT-based treatment planning to investigate whether OCT alters the DSA-based treatment planning. Furthermore, it will be investigated why the treatment planning did or did not change. For this project, 25 patients diagnosed with femoropopliteal disease and scheduled for stent placement will be included. The overview of the study is shown in the figure below.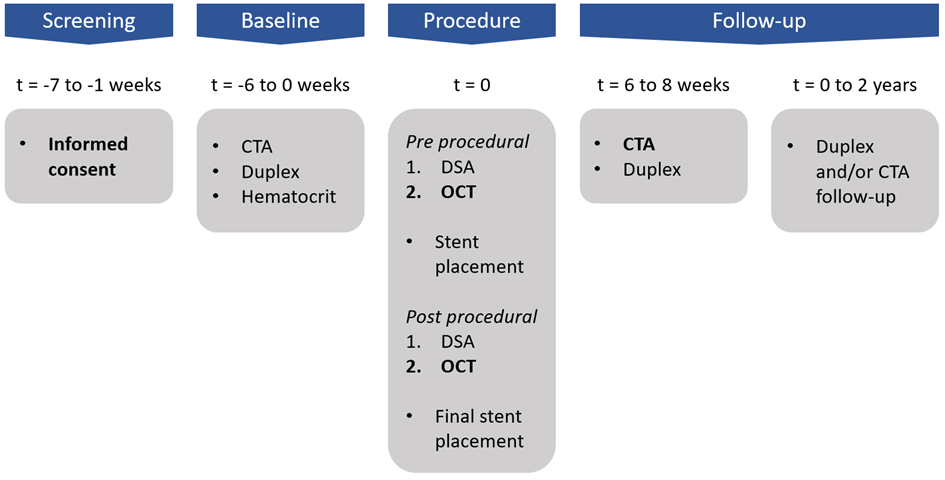
Figure 2: Schematic overview of the clinical study.
CT-A versus OCT-based CFD models
Currently, CTA scans are often used for the segmentation of the vessel lumen and obtain the geometry for the CFD simulation. However, CTA scans have a limited resolution and, in case of stents, contain blooming artefacts that could cause uncertainty in the obtained segmentation. OCT scans could also be used for the segmentation. Advantages are that the axial resolution of OCT is better and that stents do not cause blooming artefacts. It is, therefore, expected that the segmentation based on OCT scans are more patient-specific than the segmentation based on the CTA scans.
In this projects, the CTA and OCT scans obtained in the clinical study will be used to segment the vessel lumen. First, the segmentations will be compared and then the different segmentations are used as inputs for the CFD models. Then, the CFD solutions will also be compared to analyze the effect of uncertainty in the geometry on the CFD solution. If there are indeed differences, in vitro measurements will be used to investigate which imaging technique to obtain the geometry results in more patient-specific geometries.
CFD versus clinical outcome
The ultimate goal of creating the CFD models is to be able to predict in-stent restenosis and to improve the treatment of patients diagnosed with femoropopliteal disease. In this project the first steps will be taken to investigate the predictability of the CFD models that were obtained from the clinical study. Regions of disturbed blood flow and low time-averaged wall shear stress will be compared to late luminal loss, that will be obtained during the follow-up of the patients.
PUBLICATIONS
-
REFERENCES
- U. Bildirici, M. Aktas, E. Dervis, and U. Celikyurt, “Mid-Term Outcomes of Stent Overlap in Long Total Occluded Lesions of Superficial Femoral Artery,” Med. Sci. Monit., vol. 23, pp. 3130–3135, 2017.
- L. Razzouk, S. Aggarwal, F. Gorgani, and A. Babaev, “In-Stent Restenosis in the Superficial Femoral Artery,” Ann. Vasc. Surg., vol. 27, no. 4, pp. 510–524, 2013.
- W. Wijns et al., “Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN i study,” Eur. Heart J., vol. 36, no. 47, pp. 3346–3355, 2015.
- D. A. Jones et al., “Angiography Alone Versus Angiography Plus Optical Coherence Tomography to Guide Percutaneous Coronary Intervention: Outcomes From the Pan-London PCI Cohort,” JACC Cardiovasc. Interv., vol. 11, no. 14, pp. 1313–1321, 2018.
- D. Karnabatidis, K. Katsanos, I. Paraskevopoulos, A. Diamantopoulos, S. Spiliopoulos, and D. Siablis, “Frequency-domain intravascular optical coherence tomography of the femoropopliteal artery,” Cardiovasc. Intervent. Radiol., vol. 34, no. 6, pp. 1172–1181, 2011.
- W.W. Nichols, M.F. O’Rourke, and C. Vlachopoulos. McDonald’s Blood Flow in Arteries: Theoretical, experimental and clinical principles. 2011.


