PHANTOMS FOR PHOTOACOUSTIC-ULTRASOUND BREAST IMAGING
PROJECT GOAL: The projects generally aim to replicate aspects of the breast for photoacoustic-ultreasound imaging that assess the technology accurately.
FUNDED BY European Horizon 2020 PAMMOTH project under grant agreement No 732411, an initiative of the Photonics Public Private Partnership; Pioneers in Health Care (PIHC); REACT-EU
PRINCIPAL RESEARCHERS Maura Dantuma; Rianne Bulthuis; Jitse Giesen
SUPPORT Johan van Hespen, WIlma Petersen
SUPERVISOR Srirang Manohar
COLLABORATION PA Imaging R&D BV, Medisch Spectrum Twente (MST), Netherlands Cancer Institute - Antoni van Leeuwenhoek (NKI-AvL)
MOTIVATION
Imaging parameters of photoacoustic breast imaging systems are often characterized with phantoms. These objects usually contain simple structures in homogeneous media such as absorbing wires or spherical objects in scattering gels. While these kinds of basic phantoms are uncluttered and useful, they do not challenge the system as much as a breast does, and can thereby overestimate the system’s performance.
The female breast is a complex collection of tissue types, and the acoustic and optical attenuation of these tissues limit the imaging depth, the resolution and the ability to extract quantitative information. For testing and challenging photoacoustic breast imaging systems to the full extent before moving to in vivo studies, a complex breast phantom which simulates the breast’s most prevalent tissues is required.
projects
1) Semi-anthropomorphic photoacoustic breast phantom
2) Adding a simple vascular network with tunable blood oxygenation in photoacoustic breast phantom
3) A Suite of 3D test objects for performance assessment of hybrid photoacoustic-ultrasound breast imaging systems
4) Hyper-realistic breast cancer model
1) Semi-anthropomorphic photoacoustic breast phantom
We introduce the use of 3D printing of moulds to make a breast phantom and demonstrate our method to layer-by-layer build a complex PVCP breast phantom containing skin, fat, fibroglandular tissue and blood vessels. Two clusters of small blood vessels are added to mimick highly vascularized tumors. All the materials’ optical and acoustic properties are measured and presented. We demonstrate the phantom’s features in US imaging and PA tomography, and make a comparison with an in vivo PA image of a real breast. To encourage others to build this phantom, we provide the required files for 3D printing the moulds.

Fig. 1. (A-C) Models of the 3D printed moulds. A) shows the lobular shaped mould, B) the outer mould with the lobular mould placed inside. C) shows the blood vessel model that was 3D printed and subsequently embedded in silicon rubber to create a negative mould (D). (From Dantuma et al, Biomedical optics express, 10(11), 5921-5939.)
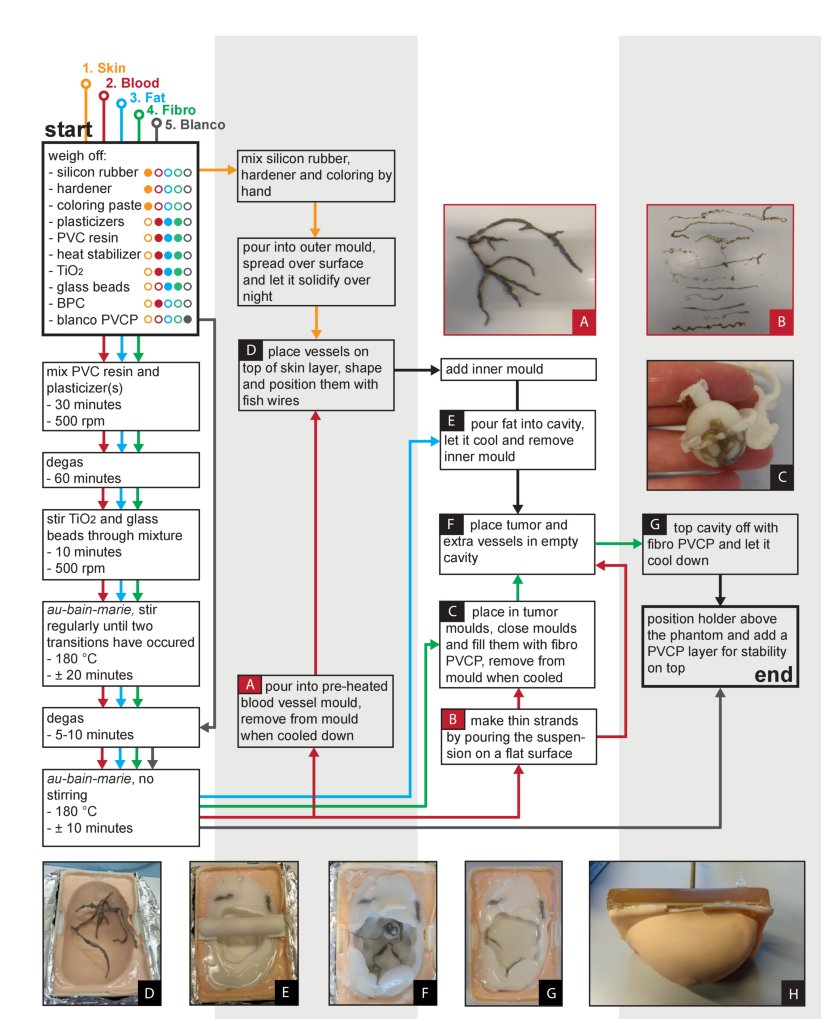
Fig. 2. Flow scheme for the development of the phantom together with pictures taken during the process. Different colors indicate the different TMMs. The numbers indicate the order of production.(From Dantuma et al, Biomedical optics express, 10(11), 5921-5939.)
2) Adding a simple vascular network with tunable blood oxygenation in photoacoustic breast phantom
Significance: Recovering accurate oxygenation estimations in the breast with quantitative photoacoustic tomography (QPAT) is not straightforward. Accurate light fluence models are required, but the unknown ground truth of the breast makes it difficult to validate them. Phantoms are often used for the validation, but most reported phantoms have a simple architecture. Fluence models developed in these simplistic objects are not accurate for application on the complex tissues of the breast.
Aim: We present a sophisticated breast phantom platform for photoacoustic (PA) and ultrasound (US) imaging in general, and specifically for QPAT. The breast phantom is semi-anthropomorphic in distribution of optical and acoustic properties and contains wall-less channels with blood.
Approach: 3D printing approaches are used to develop the solid 3D breast phantom from custom polyvinyl chloride plastisol formulations and additives for replicating the tissue optical and acoustic properties. A flow circuit was developed to flush the channels with bovine blood with a controlled oxygen saturation level. To showcase the phantom’s functionality, PA measurements were performed on the phantom with two oxygenation levels. Image reconstructions with and without fluence compensation from Monte Carlo simulations were analyzed for the accuracy of oxygen saturation estimations.
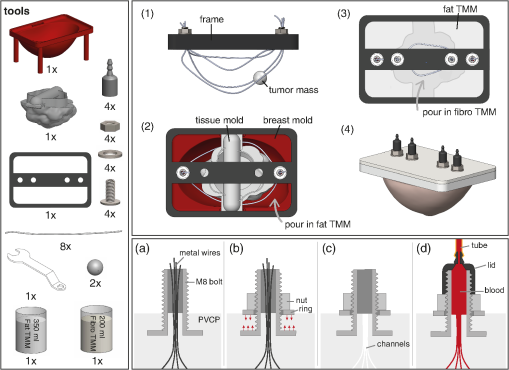
Fig. 3. Illustration of the phantom production procedure. The left panel shows all the required tools and the two right panels show steps during the production process, where the lower panel focuses on one of the connectors and shows how the channels can be connected to the flow circuit. (From Dantuma et al, Journal of biomedical optics, 26(3), 036003)
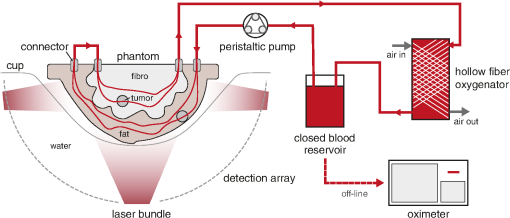
Fig. 4. The setup used during the PA measurements. The tunable oxygenation breast phantom is placed in a cup inside the Twente PAM 2 system. A peristaltic pump drives the blood from the reservoir through the circuit, where blood is being oxygenated by a hollow fiber oxygenator. Air flow through this oxygenator can be controlled to regulate the blood oxygenation. SH can be added to the blood reservoir to deoxygenate the blood. Samples are withdrawn from the oxygenator and the oxygenation is measured with the oximeter. (From Dantuma et al, Journal of biomedical optics, 26(3), 036003)
Results: We present design aspects of the phantom, demonstrate how it is developed, and present its breast-like appearance in PA and US imaging. The oxygen saturations were estimated in two regions of interest with and without using the fluence models. The fluence compensation positively influenced the SO2 estimations in all cases and confirmed that highly accurate fluence models are required to minimize estimation errors.
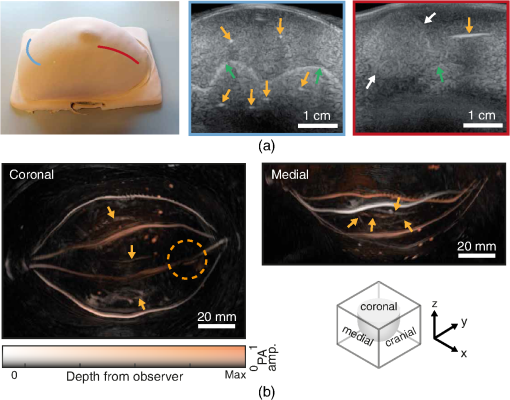
Fig. 5. (a) A picture and US images of the phantom acquired perpendicular and parallel to the channel direction. The yellow arrows highlight the channels and the green arrows highlight the interface between the fat and fibroglandular TMM. White arrows point at the tumor. The probe positions on the phantom to acquire these images are marked in red and blue lines. (b) LMIPs along the coronal, medial, and cranial plane of the reconstruction of the PA measurement with 755 nm and a 8.7% SO2. The yellow arrows point to the deeper lying vessels in the fibroglandular layer and the circle indicates the location of the shallow tumor. (From Dantuma et al, Journal of biomedical optics, 26(3), 036003)
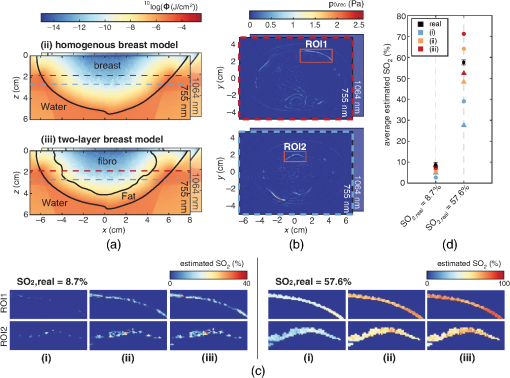
Fig. 6 Process to recover SO2 values, with (a) the fluence compensation approaches where (ii) and (iii) show the fluence models at y=0, with the contours of the tissue volumes where the MC simulations were performed on. (b) Two slices of the reconstructed pressures of the measurement with the 57.6% SO2. The ROIs used for the SO2 estimations are highlighted. The MIPs of the final SO2 estimations for both ROIs and both measurements are presented in (c) and the found average SO2 values of for ROI1 (circles) and ROI2 (triangles) are plotted in (d).(From Dantuma et al, Journal of biomedical optics, 26(3), 036003)
Conclusions: This phantom allows studies to be performed in PA in carefully controlled laboratory settings to validate approaches to recover both qualitative and quantitative features sought after in in-vivo studies. We believe that testing with phantoms of this complexity can streamline the transition of new PA technologies from the laboratory to studies in the clinic.
3) A Suite of 3D test objects for performance assessment of hybrid photoacoustic-ultrasound breast imaging systems
Significance: During the development and early testing phases of new photoacoustic (PA) breast imaging systems, several choices need to be made in aspects of system design and measurement sequences. Decision-making can be complex for state-of-the-art systems such as 3D hybrid photoacoustic-ultrasound (PA-US) breast imagers intended for multispectral quantitative imaging. These systems have a large set of design choices and system settings that affect imaging performance in different ways and often require trade-offs. Decisions have to be made carefully as they can strongly influence the imaging performance.
Aim: A systematic approach to assess the influence of various choices on the imaging performance in carefully controlled laboratory situations is crucial before starting with human studies. Test objects and phantoms are used for first imaging studies, but most reported structures have a 2D geometry and are not suitable to assess all the image quality characteristics (IQCs) of 3D hybrid PA-US systems.
Approach: Our work introduces a suite of five test objects designed for hybrid PA-US systems with a 3D detection aperture. We present the test object designs and production protocols and explain how they can be used to study various performance measures. To demonstrate the utility of the developed objects, measurements are made with an existing tomographic PA system.
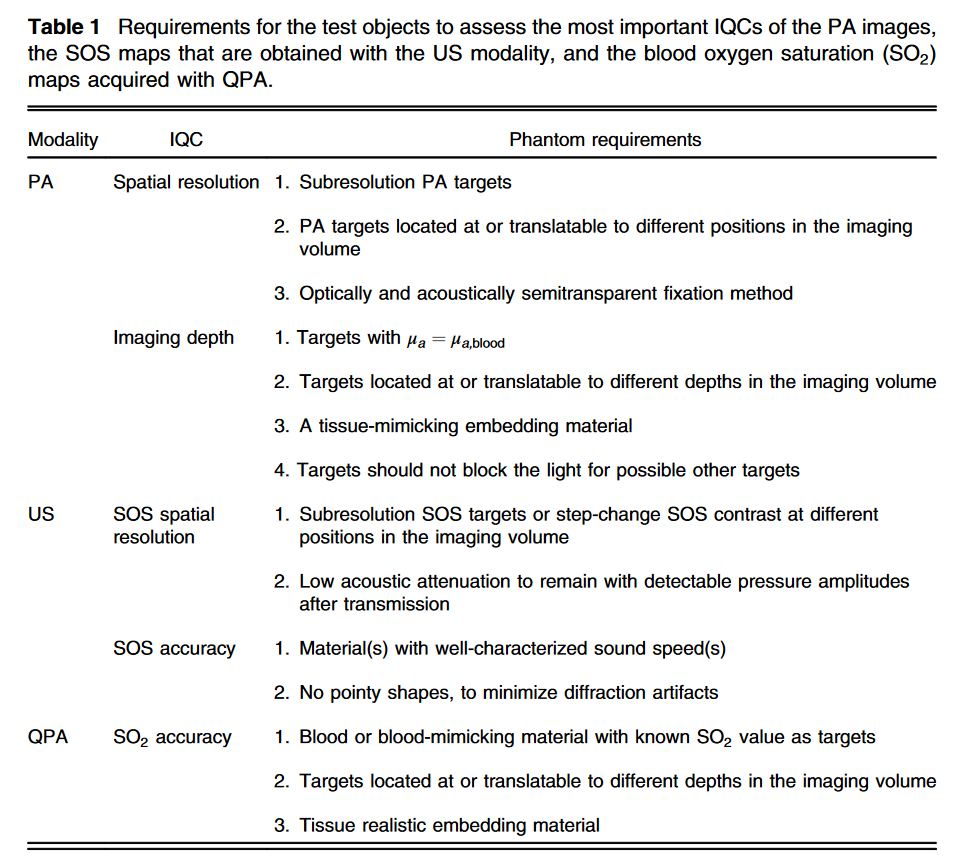
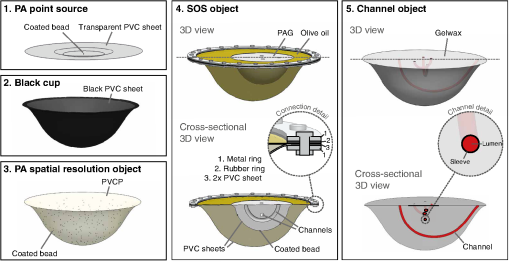
Fig. 7 Illustrations in 3D of the test object suite showing their most important features. PA, photoacoustics; PVC, polyvinylchloride; PVCP, polyvinylchloride plastisol; and PAG, polyacrylamide gel. (From Dantuma et al, Journal of biomedical optics, 27(7), 074709.)
Results: Two test objects were developed for measurements of the US detectors’ impulse responses and light distribution on the breast surface. Three others were developed to assess image quality and quantitative accuracy of the PA and US modes. Three of the five objects were imaged to demonstrate their use.

Fig. 8 Photographs of test objects. (a) The single PA source attached to a PVC sheet. A bright-field microscopy image of the bead is shown in the inset. (b) One of the eight produced black cups (size 6). A metal ring is attached to the cup to mount it in the imaging aperture. (c) The PA spatial resolution object, showing the PVCP layers. (From Dantuma et al, Journal of biomedical optics, 27(7), 074709.)
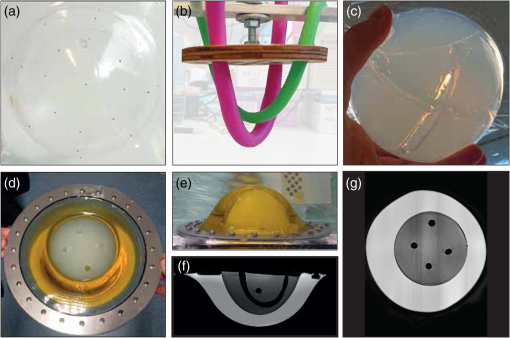
Fig. 9 Pictures taken during and after the production of the SOS object. (a) Subresolution beads glued to the ellipsoid cup. (b) Modeling balloons filled with water as placeholders for the channels in the PAG. (c) The polyacryl amide gel volume after draining and removing the balloons. (d), (e) Top view and side view of the assembled SOS object, showing the olive oil and PAG compartments. The channel openings in the PAG are also visible. (f), (g) Cross sections of an MRI scan made of the object, showing the object’s build-up. (From Dantuma et al, Journal of biomedical optics, 27(7), 074709.)

Fig. 10 Pictures taken during and after production of the channel object. (a) The four bent metal wires covered in the US sleeves. (b) The channel shapes positioned in the cup eight shaped mould using a holder. (c) Top view of the channel phantom after pouring in the gel wax and removal of the holder and the metal wires. The channel sleeves protrude from the object and can optionally be connected to a flow circuit.(From Dantuma et al, Journal of biomedical optics, 27(7), 074709.)
Conclusions: The developed test objects allow one to study influences of various choices in design and system settings. With this, IQCs can be assessed as a function of measurement sequence settings for the PA and US modes in a controlled way. Systematic studies and measurements using these objects will help to optimize various system settings and measurement protocols in laboratory situations before embarking on human studies.
CONTACT
PUBLICATIONS
- Kharine, A., Manohar, S., Seeton, R., Kolkman, R. G., Bolt, R. A., Steenbergen, W., & de Mul, F. F. (2003). Poly (vinyl alcohol) gels for use as tissue phantoms in photoacoustic mammography. Physics in Medicine & Biology, 48(3), 357.
- Xia, W., Piras, D., Heijblom, M., Steenbergen, W., Van Leeuwen, T. G., & Manohar, S. (2011). Poly (vinyl alcohol) gels as photoacoustic breast phantoms revisited. Journal of biomedical optics, 16(7), 075002.
- Dantuma, M., van Dommelen, R., & Manohar, S. (2019). Semi-anthropomorphic photoacoustic breast phantom. Biomedical optics express, 10(11), 5921-5939
- Dantuma, M., Kruitwagen, S., Julia, J. O., van Meerdervoort, R. P. P., & Manohar, S. (2021). Tunable blood oxygenation in the vascular anatomy of a semi-anthropomorphic photoacoustic breast phantom. Journal of biomedical optics, 26(3), 036003.
- Dantuma, M. M., Kruitwagen, S. C., Weggemans, M. J., Op't Root, T. J., & Manohar, S. S. (2021). Suite of 3D test objects for performance assessment of hybrid photoacoustic-ultrasound breast imaging systems. Journal of biomedical optics, 27(7), 074709.
.

