This research is performed by: Nicolas Hildenbrand
Daily supervisor: Bernard Boukamp
Several solid inorganic oxide systems show appreciable conductivity for oxygen ions. An important application of this property is found in the so-called Solid Oxide Fuel Cells (SOFCs). These SOFCs can transform chemical energy directly into electrical energy with high efficiency and without pollution. The basic SOFC consists of three different layers of inorganic materials: Two electrodes, which may consist of composite materials, separated by a ceramic electrolyte.
At one electrode, the cathode, atmospheric oxygen (O2) is transformed into oxygen ions (O2-) which then move through the electrolyte to the other electrode, the anode, where they react with the fuel. In this process electrons are forced through an external (electrical) load. The working principle is schematically sketched below.
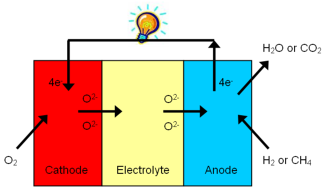
Figure 1. Working principle of a Solid Oxide Fuel Cell (SOFC).
Recently, there has been much focus on the development of cathode and electrolyte materials, allowing operation in the temperature range of 600°C. These relatively low temperatures enable the utilization of alloys as interconnect materials vs. ceramics, thus lowering cost and extending cell life.
Perovskite cathodes from the La1-xSrxCo1-yFeyO3-z family have already shown excellent performance due to the high electronic and ionic conductivity. The major drawback is the high reactivity with the standard used yttrium-doped zirconia typ electrolytes. The use of yttrium-doped ceria interlayers indicates that this reactivity might be suppressed.
In IMS we study the fundamental material properties (electronic conductivity, ionic conductivity, surface oxygen exchange rates and catalytic properties) to optimize the electrode resistance through proper control of the electrode microstructure.
Furthermore, attention will be paid to the degradation and possible chromium poisoning of the electrodes. This will be performed in cooperation with Calipso B.V. in Eindhoven.
Preparation of the electrodes will be performed in cooperation with ECN (for porous electrodes) and within the IMS group by PLD. Characterisation of these electrodes will be done with standard electrochemical research methods (dc and ac). Surface catalytic and oxygen surface exchange properties will be studied with 18O-exchange experiments.
