Introduction
Dry ice is of fundamental importance in a wide variety of technical applications ranging from cleaning surfaces of very large telescopes, spray cooling, refrigeration, to biological processes like cryopreservation. The triple point temperature and pressure of dry ice are above normal ambient temperature and pressure, causing only the solid and gaseous phase to exist at atmospheric conditions, and the solid phase to continuously sublimate. Since dry ice is in permanently sublimating state at standard ambient condition, it exhibits interesting behaviour involving coupled heat, mass and momentum transport phenomena. The aim of this PhD project is to understand physical mechanisms of sublimation involving two phase flows. In particular, the problem is divided into two categories, namely, (i) dry ice sublimation in free ambient with varying conditions, and, (ii) dry ice sublimation when it is in contact with a substrate of varying thermal properties. A brief description of the research activities undertaken in the PhD project is summarised below.
Saturation temperature of solid carbon dioxide for varying boundary conditions
A common misconception about the basic understanding of dry ice temperature exists in industries as well as academia. The dry ice sublimation temperature of -78.5 °C / 194.65 K is widely reported in literature and used in industries. This value is only conditionally true, i.e. in an environment of saturated CO2 vapor in equilibrium with dry ice at 1 bar pressure. But in real applications dry ice utilised in an unsaturated environment which results in dry ice temperature lower than -78.5 °C because of CO2 diffusion in the unsaturated environment. In order to correct this widespread misconception, a well-controlled experimental setup was devised to accurately estimate the influence of different environments with varying pressure and CO2 concentration on dry ice temperature. Figure 1 shows a dry ice sphere suspended on a thermocouple in a controlled ambient. Based on the measurements, it is shown that dry ice temperature can drop below -98 °C / 174 K in normal atmosphere condition. This deviation of 20 K from the value normally reported in literature is of significance for modelling different thermal processes involving dry ice and to gain deeper insights into the sublimation process.
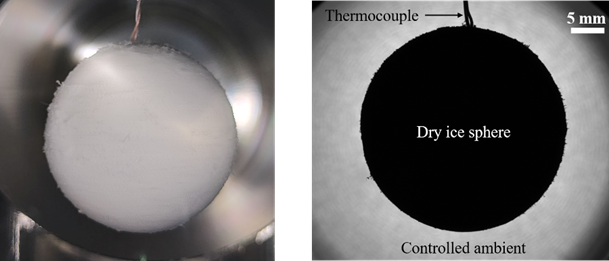
Figure 1: A dry ice sphere suspended on a thermocouple in a controlled environment (left); A backlight image of a dry ice sphere captured during an experiment (right)
Leidenfrost dynamics of sublimating solid carbon dioxide
When placed on a hot surface, dry ice that sublimates at atmospheric conditions, hovers on a cushion of its own vapor in the Leidenfrost state, see schematic in Figure 2 . Discovered in 1756, the Leidenfrost is mostly studied for liquids in contact with solid and liquid substrates. In this work, we investigated this phenomenon experimentally and theoretically for dry ice pellets on a hot sapphire substrate. While studying Leidenfrost effect, a key parameter for estimating the vapor pressure, heat transfer rate and the flow profile in the vapor layer is its thickness. A direct measurement of vapor layer thickness below dry ice pellet, and a systematic comparison with theoretical model is missing in the literature. For the first time, the Optical Coherence Tomography technique is adapted to measure vapor layer thickness and gain insights into the Leidenfrost phenomenon at a high temporal and spatial scale. Time evolution of vapor layer thickness, vapor layer profile and effects of key parameter like substrate temperature on the vapor layer thickness is measured and compared with the results of the phenological model based on lubrication theory.

Figure 2: Schematic considered for the formulation of theoretical model for Leidenfrost
Dry ice sublimation in an insulation box
Dry ice sublimation inside a polystyrene foam package is experimentally and numerically investigated considering macro scale conductive and radiative heat transfer processes. Because of the large latent heat of sublimation of dry ice (and small Stefan number), the front of dry ice inside the box moves very slowly. Based on this assumption, a three dimensional quasi-steady model is developed to predict variation of dry ice mass inside the insulation packages. The model uses an iterative approach in which the dry ice level is changed in every subsequent step until the dry ice interface reaches the bottom surface of the insulation package, see fig. The model is validated against mass and temperature measurements performed on two types of insulation packages made of different material and geometry. The model is able to fairly predict the reduction in dry ice mass over time and the end-of-sublimation time. Both experimental and numerical results show that the sublimation rate of dry ice can be reduced by covering the inner walls of insulation package with a reflective layer like aluminized mylar foil. The good agreement between model and experimental data of dry ice mass variation makes it possible for application engineers to use the modeling approach in estimating sublimation rates of dry ice inside a insulation package.
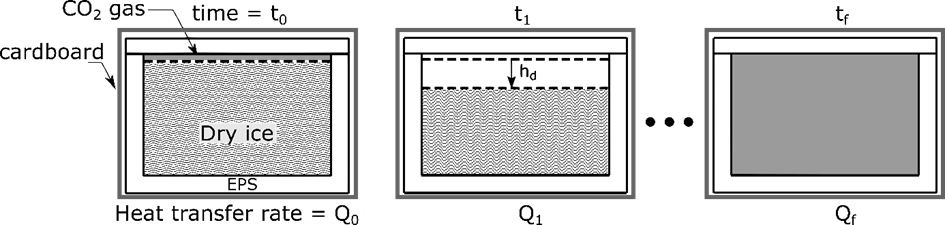
Figure 3: Front view cut section of the EPS box is shown at different instance of time. Q represent the heat transfer rate that goes into the EPS box. In the schematic, dry ice domain is represented by shaded area. The colors white and grey represent expanded polystyrene and carbon dioxide gas, respectively
Contact: Abhishek Purandare
