Introduction to wavefront shaping
Bring me to the available projects!
Scattering of light forms a huge limitations for optical imaging, but also severely limits telecommunication, spectroscopy, and other optical techniques. In the last few decades, a tremendous effort was put in developing imaging methods that work inside turbid biological tissue. That research has brought forward important new imaging methods like optical coherence tomography, diffusion tomography and laser speckle velocimetry.
Roughly, all optical methods can be divided into three subclasses:
- High-resolution methods using ballistic light. Ballistic light is the part of the light that is not scattered and propagates through the tissue along a straight ray. Methods that use ballistic light include all types of microscopes (including fluorescence microscopes and multi-photon microscopes), as well as optical coherence tomography and low coherence spectroscopy. Ballistic light forms high resolution images (typical resolution < 1 µm). However, the amount of ballistic light decreases exponentially with depth, so the penetration depth of even the most advanced microscopes is limited to about a millimeter.
- Low-resolution methods using scattered light. When light is scattered inside the tissue, you can no longer tell where it came from exactly. However, you can still estimate the position of light sources and optical properties of the tissue using techniques like diffuse optical tomography. Such methods are able to image up to a depth of several centimeters, albeit at a severely reduced resolution (typically 1 millimeter or worse).
- Ultrasound-assisted methods. Methods like photoacoustic imaging and acousto-optic tomography combine the advantages of ultrasound and light. In these methods, the image contrast is given by the optical absorption (and other optical properties) of the tissue, whereas the resolution is determined by the ultrasound system. Since biological tissue hardly scatters ultrasound, the resolution of such hybrid methods is much higher than for diffuse optical methods (typically better than 1 millimeter).
In 2007, an entirely different approach was demonstrated by Ivo Vellekoop and Allard Mosk (see doi:10.1364/OL.32.002309). In this approach, called wavefront shaping, the incident light beam is modified in such a way that the scattered light converges to a chosen point inside the scattering material (see figure)
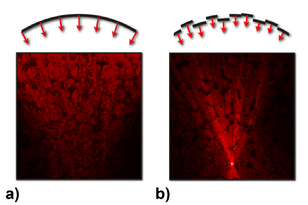
a) When you try to use a lens to focus light inside tissue, the light gets scattered and no focus is formed.
b) By shaping the wavefront of the incident light, the scattered light can be directed towards a focus at any desired position.
The idea of wavefront shaping is to use a spatial light modulator to construct a wave that exactly matches the microscopic scattering properties of the object. At this point, the scattered waves interfere constructively, generating a sharp and intense focus.
Finding the matching wavefront for focusing at a given point is far from trivial: a different wave is needed for each sample and for each point in that sample. Since 2007, many approaches have been investigated, including feedback from fluorescently labelled nanobeads, ultrasound tagging, photoacoustic feedback, focusing on moving objects, second-harmonic generation, and many more. So far, none of the approaches are completely suitable for biomedical imaging yet. Finding better approaches and improving existing ones currently is the subject of intense research.
In conclusion, it is possible to focus light sharply deep inside scattering tissue, if ‘only’ you know the matching wave to send in. Such a focus can be used for imaging (for instance, raster scanning fluorescence microscopy), potentially achieving sub-micrometer resolution up to a depth of several centimeters, enabling microscopy through the intact skin. In addition, one could use the intense focus to manipulate or kill individual cells. Therefore, we are researching new and improved ways of focusing light deep inside tissue and using scattered light for high-resolution imaging.
