Fast-charging batteries
Modern life demands batteries that are not only energy efficient but also fast to charge, affordable, and safe. Achieving this starts from the very materials batteries are made of. “We focus on further developing known battery materials and exploring new ones that can store more energy. Our research integrates all battery components—the anode, cathode, and electrolyte—as we aim for holistic improvement,” Prof. Mark Huijben, whose research focuses on materials science specifically for batteries, says.
Energy density, or how much energy a battery can store, has long been a metric for material selection. But as consumer expectations shift, the ability to charge faster becomes just as important. There are a lot of materials that can store a large amount of energy well but when charged too quickly, they degrade faster. “We focus on why this degradation happens and explore materials that can better handle fast charging without breaking down,” Huijben explains.
Niobate anode
Huijben's research group has experimented with so-called niobate anodes. A new alternative to a graphite anode, which is used in traditional batteries. An anode is the minus side of a battery and a cathode is the plus side. Niobate is resistant to fast charging. If niobate is scaled up to industrial production, it can reduce charging times by a factor of 10, making electric cars, for example, much faster to charge.
Ethical and safer batteries
Besides improving charging speed, there is a growing need for more sustainable and ethical materials, particularly for cathodes. They usually contain cobalt or nickel—scarce materials linked to environmental harm and human rights abuses. “We are exploring alternatives that don't use these elements but iron and manganese instead. Also, we are shifting from lithium batteries to sodium batteries. Sodium is globally accessible. Its use could lower battery costs even though it comes with a somewhat reduced energy storage,” Huijben says.
Another important focus is safety. Between the anode and cathode lies the electrolyte. Most batteries have liquid electrolytes, which are essential for bringing the lithium from one side to the other and back during charging and discharging. However, liquid electrolytes are highly flammable, posing risks of overheating, and in extreme cases, fire.
The Huijben research group in collaboration with industry partners is exploring solid compounds that could replace the liquid in batteries for maritime transport, heavy machinery and similar applications. “Solid-state batteries are intrinsically safer and are a promising alternative we expect to come to the market very soon.”
Battery Centre Twente
To move toward sustainable batteries, it is crucial to consider the entire production process. “How are factories designed? How efficient is the manufacturing? We need to address every link in the value chain,” Huijben says. The Battery Centre Twente brings researchers and companies together to optimise the battery lifecycle at every step: materials sourcing, green energy use, manufacturing techniques, including automation and software development, and recycling of the original materials.
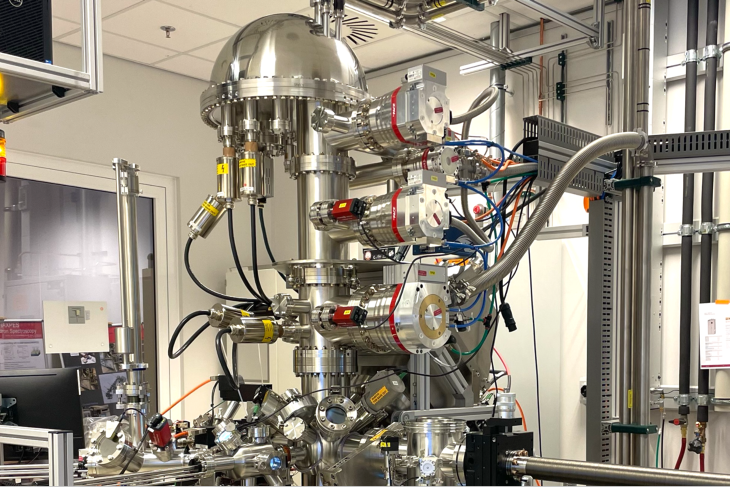
“The biggest challenge in our work is having access to equipment that enables us to conduct those atomic-scale analyses. Thanks to the MESA+ NanoLab, we can see chemical reactions within a battery during charging and discharging, understand why one material performs better than the other, and identify the causes of degradation to prevent it. This allows us to take battery performance to a much higher level.”
Photo: The unique HAXPES analysis system in the MESA+ Nanolab. A one of a kind system. More info.