Motivation
Since the industrial revolution, approximately 30% of the anthropogenic CO2 released into the atmosphere is absorbed by oceans [1]. As a result the ocean’s pH drops (ocean acidification) and a huge ecosystem is affected. In particular, a specific algae, named coccolithophores, will be affected. They play an important role in the ocean’s carbon fixation capacity. In some regions, these algae are responsible for more than 20% of the total carbon fixation by means of photosynthesis and calcification [2]. Coccolithophores form a calcite (calcium carbonate) exoskeleton, which causes the coccolithophore to sink into the deep ocean while sequestrating carbon, as illustrated in figure 1. In a recent report [3], the European Academies’ Science Advisory Council (EASCAC) also discusses the possibility to fertilize the oceans and thereby increase the CO2 uptake by phytoplankton and other micro plants in order to realize negative CO2 emission. At this point, still a lot of uncertainties and risks are involved. More research must be done to get more insight into this complex ecosystem in order to predict if coccolithophores or their genetic variations can be deployed as negative emission technology (NET).
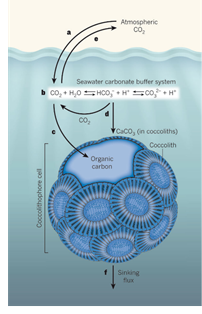
Figure 1: Borrowed from [4]
Lab-on-a-Chip technology
Lab-on-a-Chip (LoC) technology provides a novel platform to obtain more insight in coccolithophores and their role in carbon sequestration. LoC has several advantages over traditional research under bulk conditions performed by biologists such as enabling the study of single cells as a function of local parameters like temperature, CO2 concentration and pH.

Figure 2: SEM image by Darya Hadavi, Nanolab UTwente
Goal
One of the challenges in this research involves one of the most crucial factors in the sequestration of CO2 by coccolithophores: the ratio between its organic and inorganic composition. The cell growth (particulate organic carbon (POC)) is the result of photosynthesis and stimulates the local uptake of CO2, whereas the formation of calcite (particulate inorganic carbon (PIC)) by calcification releases CO2 and makes the cell heavier and sink. A chip needs to be developed to measure on chip the PIC:POC ratio of single cells.
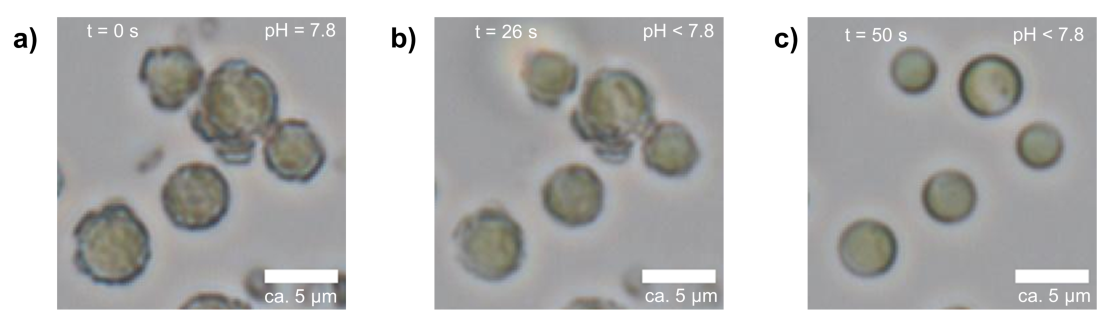
Figure 3: Dissolution of the calcite exoskeleton of coccolithophore over time, due to acidification of the medium.
Research Challenges: Electrical Impedance Spectroscopy
Currently, we are working on a method called electrical impedance spectroscopy to study the PIC:POC ratio of single cells. So far we have been able to differentiate between calcified and completely decalcified cells. We have plenty of ideas for follow-up research:
- Differential electrical impedance measurements to improve the sensitivity of our chip
- The use of Neural Networks to differentiate between calcified and decalcified cells
- Investigation of the relation between electrical opacity and particle height in the microfluidic channel
Do want to help solving the challenges we currently face? Please contact us!
Albert van den Berg
a.vandenberg@utwente.nl
Wouter Olthuis
w.olthuis@utwente.nl
Douwe de Bruijn
d.s.debruijn@utwente.nl


[1] C. L. Sabine, R. A. Feely, N. Gruber, R. M. Key, and B. and others L. K. B. J. L. and W. R. and W. C. and W. Douglas WR and Tilbrook, “The Oceanic Sink for Anthropogenic CO2,” Science (80-. )., vol. 305, no. 5682, pp. 367–371, 2004.
[2] A. J. Poulton, T. R. Adey, W. M. Balch, and P. M. Holligan, “Relating coccolithophore calcification rates to phytoplankton community dynamics: Regional differences and implications for carbon export,” Deep. Res. Part II Top. Stud. Oceanogr., vol. 54, no. 5–7, pp. 538–557, 2007.
[3] European Academies’ Science Advisory Council, Science Advice for the Benefit of Europe Negative emission technologies: What role in meeting Paris Agreement targets?, no. 35. 2018.
[4] D. A. Hutchins, “Oceanography: Forecasting the rain ratio,” Nature, vol. 476, no. 7358, pp. 41–42, 2011.
