Microfluidics high throughput production of hollow microgels
to create a next-generation 3D stem cell organoid platform
Stem cell organoids have consistently been demonstrated to outperform traditional cultures in terms of tissue formation, both in vitro and in vivo. This makes these organoids highly interesting for tissue engineering and drug screening purposes. In recent years, we and others have developed microfabrication techniques that allow us to form organoids in microwell arrays. However, for tissue engineering purposes, all these techniques are hindered by a major limitation: they require an in vitro step of at least 24 hours to create these microaggregates. This limits the translation application as it requires two invasive steps on separate days: one to obtain the cells and another to reimplant the cells. This hinders clinical translation and substantially increases the cost of an envisioned therapy.
In this project, we will develop an approach that allows the microaggregation to take place within the patient’s own body (in situ). By exploiting a recently developed microfluidic microgel production platform, we will be able to controllable produce hollow cell laden micrometer sized hydrogels (microgels). The cells in the hollow cavity of the microgel will then aggregate into organoids within the microgels. Advantageously, the aggregation can thus take place inside of the body. In other words, our approach would unique enable the isolation and reimplantation of stem cells in a single step. This will reduce the treatment’s cost and expedite the clinical translation of organoid technology. Moreover, having the organoids within a biomaterial coating also grants unique opportunities to provide the organoids with experimenter-controlled microenvironments, which will improve our control over stem cell fate and on eventual clinical outcomes.
Specifically, you will be involved in the microfabrication of our microfluidic platforms, optimizing the production of hollow microgels, expanding stem cells, and forming organoids within the hollow micromaterials, and perform high-end analyses on the formed constructs. This project will therefore provide aspiring students with an interdisciplinary skillset and knowledge framework on microfabrication, microfluidics, micromaterials, organoids, stem cells, and tissue engineering.
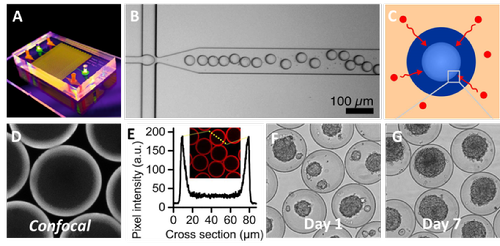
Figure 1: Pilot experiments illustrating (A) newly fabricated microfluidic device, (B) which was used to create microgels. (C) These microgels were crosslinked outside-in using a technique that leaves the core liquid, (D-E) which was demonstrated using fluorescent confocal microscopy. (F) The cells within the microgel’s hollow core rapidly microaggregated in vitro, (G) and proliferated over time indicating a high cytocompatibility.
