- PFAS removal using bio-based sorbents
In our everyday lives we frequently use products which contain fluorinated chemicals (non-stick cookware, cosmetic products, water resistant clothing, ect.). In order to produce these fluorinated coatings the industry uses poly- or perfluorinated alkyl substances (PFAS) in their processes. Both the waste streams from these production processes and excretion from these products themselves leads to these PFAS entering our water supplies. Many of the problematic molecules in this class of compounds (containing >9000 different compounds) contain a charged head group giving rise to high solubilities in water combined with a very stable carbon-fluorine tail. The strength of the fluorine-carbon bond means that these compounds are non-biodegradable and they will therefore ‘almost’ indefinitely build up inside of the water system. For this reason these compounds are also often referred to as the ‘forever chemicals’.
In recent years there is increasing concern that the ubiquitous presence of these PFAS in water resources is not addressed by current water treatment facilities and therefore they end up in drinking water. Currently the only widely employed method for removal of PFAS from water is adsorption onto activated carbons [1], however the use of activated carbons seems to be unsustainable from both a economical perspective and a environmental perspective. In the Netherlands the concern about these compounds has consistently shown up in the news over the past couple of years, although many regions in the USA [2] and China [3] are substantially more severely impacted.
In this project you will use bio-based materials of different origins and test their ability to adsorb PFAS from water. Both batch testing and flow through testing will be employed to asses adsorption capacity and kinetics at environmentally relevant concentrations. Furthermore you will need to characterise the adsorbents to be able to make a fair comparison. Fitting of adsorption models, statistical analysis and choices in data presentation will be instrumental to make your results both relevant and meaningful. This is a very active field of study, so your view on what practises are correct and which are less relevant is very valued. The assignment is suitable for students of universities (both bachelors and masters level) and students of universities of applied sciences.
This assignment is part of a project to develop a new process to treat water resources plagued by PFAS to make drinking water that is safe for consumption. The data you collect will be directly used for the future direction of the project!
If you are interested in this assignment please contact Jurgen Roman (j.b.roman@utwente.nl).
[1] Xiao, X., Ulrich, B. A., Chen, B., & Higgins, C. P. (2017). Sorption of Poly- and Perfluoroalkyl Substances (PFASs) Relevant to Aqueous Film-Forming Foam (AFFF)-Impacted Groundwater by Biochars and Activated Carbon. Environmental Science and Technology, 51(11), 6342–6351. https://doi.org/10.1021/ACS.EST.7B00970
[2] Johnson, C. D. (2022). Per- and Polyfluoroalkyl Substances: A Preliminary Evaluation of Groundwater Contamination in the Western States. https://www.osti.gov/biblio/1879543
[3] Liu, L., Qu, Y., Huang, J., & Weber, R. (2021). Per- and polyfluoroalkyl substances (PFASs) in Chinese drinking water: risk assessment and geographical distribution. Environmental Sciences Europe, 33(1), 1–12. https://doi.org/10.1186/s12302-020-00425-3
- MOFs for specific ion selective membranes
Metal-organic frameworks (MOFs) are a class of porous hybrid materials that are composed of coordinated metal atoms that are linked to each other via organic linker molecules. This forms porous materials with pores in the order of magnitude of several Angstroms. This property makes MOFs interesting to use as a membrane to separate specific ions from each other, unlike conventional ion-exchange membranes which are primary based on charge-exclusion and show very limited ion-ion selectivity.
Relatively recently, in the field of ion separations in water MOFs became of interest for specific water treatment that needs to target specific types of ions in water that also consists of other less interesting ions that do not need to be removed. Due to these small pore sizes, ions that are transported through the membrane have to dehydrate some shells of water to fit into the MOF pores. Therefore MOF based membranes can separate ions based on how easy they lose their water shells[1,3]. This makes it possible for instance to recovery lithium ions selectively from a saline feed stream and it is possible to create a membrane that is more selective towards monovalent ions compared to multivalent ions (Cl- vs. SO4- or Na+ vs Mg2+) [1,2]. Furthermore it was found specifically for fluoride ions, that specifically bind fluoride to the MOF structure also enhanced the transport through a MOF membranes [3].
In this project we will make MOF modified membranes and will characterize the membrane for instance with SEM and XRD. Furthermore we will analyze the modified ion transport through the MOF membrane with bi-ionic potential measurements and finally we can potentially test how well this membrane works in an electric-driven separation processes to separate specific ions. This project has industrial implications into developing new resource recovery processes in industry of valuable components, such as lithium from seawater.
This assignment can be suitable for either bachelor or master students.
If you’re interested and would like to know more about this project don’t hesitate to contact Harm Wiegerinck
Email: h.t.m.wiegerinck@utwente.nl
[1]:J. Lu et al. Efficient metal ion sieving in rectifying subnanochannels enabled by metal–organic frameworks. Nature Materials 2020 p. 767-774
[2]: H. Zhang. Ultrafast selective transport of alkali metal ions in metal organic frameworks with subnanometer pores. Science Advances 2018 vol. 4 (2)
[3]:X.Li. Fast and selective fluoride ion conduction in sub-1-nanometer metal-organic framework channels. Nature Communications 2019 vol. 10 (1)
- Effect of a temperature & concentration gradient on the transport of ions through ion selective porous media
Previously, we have researched the influence of temperature gradients on the performance of electrodialysis experimentally [1,2] as well as numerically with a model system of ion selective nanochannels [3]. It was found that temperature differences can increase current at a given voltage (as expected from conductivity) and more interestingly that ion selectivity could be tuned based on these temperature differences due to variation in how the hydrated radius of ions change at different temperatures.
However, in an electrodialysis process, the salt concentration changes along the length of the membrane due to the electrical field, which drives the ions through the ion-exchange membrane. Therefore, more fundamental research into the effect of temperature and concentration gradients on the transport rate of ions through a membrane is necessary to exploit these effects.
In this work, we plan to study ion and related transport phenomena through a bed packed with ion-exchange resin particles, while keeping the temperature gradient and concentration gradient nearly constant across the bed. Ion exchange resin particles are composed of charged groups and therefore will preferentially allow the transport of counterions through the bed, while co-ions are retained by the ion exchange resin, which mimics a ion-exchange membrane. This setup allows to study the effect of concentration difference, electrical field strength, etc. on the rate of transport of different ions through porous ion exchange beds. This then has possible implications in how to improve industrially scale ion-exchange processes using low-grade waste heat.
This assignment can be suitable for either bachelor and master students
If you’re interested and would like to know more about this project don’t hesitate to contact Harm Wiegerinck
Email: h.t.m.wiegerinck@utwente.nl
[1]A. Benneker et al. Effect of temperature gradients in (reverse) electrodialysis in the Ohmic regime. Journal of Membrane Science 2018 pg.421-428
[2]: A.Benneker et al. Influence of temperature gradients on mono- and divalent ion transport in electrodialysis at limiting currents. Desalination 2018 pg. 62-69
[3] A. Benneker et al. Influence of temperature gradients on charge transport in asymmetric nanochannels. Physical Chemistry Chemical Physics 2017 vol. 19 (41)
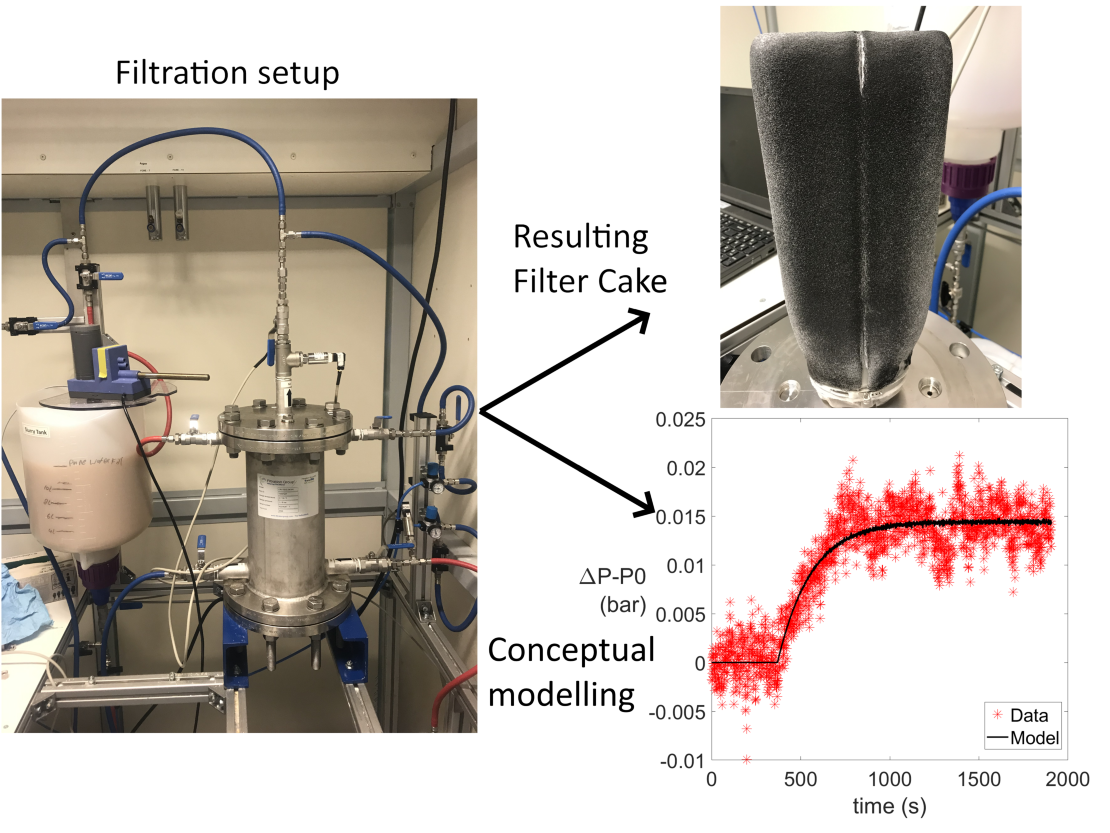
- Cake filtration using a Cricketfilter®
Background
Cake filtration is an old and established separation method for separating solids from fluids, with wide ranging applications in industry[1]. Common applications are found in chemical, petrochemical, pharmaceutical, mining and food industries[2]. Besides the obvious application of separations of solids from suspensions (e.g. recycling of catalysts), also the adsorption of compounds (e.g. discolouration of olive oil by bleaching earth) is also an important use case for cake filtration. The combined removal of small particulate matter through filtration and unwanted chemicals due to adsorption leads to an efficient integrated process step.
Although the basic concepts of cake filtration are easily understood, it is a complex process with many interconnected variables. Still over the long lifespan of this process many developments have been made, both in filter engineering and understanding of the process[2]. However in industrial design is still complex, partly due to a large variety of feed stocks and applications. Therefore there is a lot to study from both a chemical engineering and physics perspective.
One of such developments is the Cricketfilter® element developed by Amafilter. It is an evolution of the classic vertical ‘candle filter’ element, in which the internal volume is reduced to maximise the available filtration area in a given vessel volume.
Master thesis assignment
In this assignment the focus is on describing the cake filtration process in a vessel with a Cricketfilter® element. Investigating the particle properties (e.g. size, shape and charge) and relating this to the results obtained from filtration cycles leads to improved understanding of the process and possible prediction of future filtration performance. In order to do this a coupling of the possible interactions between the particles in different conditions (repulsion and agglomeration as can be described by DLVO or physical linkage or hindrance due to particle strucure) to the resulting filtration performance and cake structure will need to be undertaken. Once an understanding of the build-up of cake material on the Cricketfilter® element has been established, possible use cases such as adsorption of micropollutants in the solid material of a cake layer can be investigated. Finally the dynamic cake filtration process also leads itself well to an analytical/numerical approach to gain further insights into the development of the cake layer under varying conditions.
The following aspects are of high interest:
- Experimental investigation of the operation of the cake filtration process on the Cricketfilter® element, using various materials (and combinations thereof) and operating conditions.
- Determining relations between the properties of the materials used, their filtration performance and finally the resulting cakes obtained.
- An analytical/numerical description of the cake/cake build-up
- Experimental investigation into the use of a cake layer for the adsorption of contaminants from an aqueous phase
If you are interested in this assignment please contact Jurgen Roman (j.b.roman@utwente.nl).
[1] Tien, C. (2002). Cake filtration research—a personal view. Powder Technology, 127(1), 1–8. https://doi.org/10.1016/S0032-5910(02)00063-3
[2] Khean, T. S. (2003). Studies in filter cake characterisation and modelling. https://core.ac.uk/works/9093340