Bubbles are known to influence energy and mass transfer in gas evolving electrodes. Many electrochemical reactions produce gas that can lead to bubbles forming at the reaction site. Those bubbles can reduce the efficiency of reaction which leads to energy losses. David Fernandez Rivas, and his collaborators of the University of Twente and New York University, published different strategies to mitigate losses or even exploit bubbles in the journal Joule.
In the article, Fernandez Rivas explains that bubbles are formed in plenty of large industrial electrosynthesis processes like the reduction of CO2 to produce organic fuels. That is a problem in the current trend of electrification of the chemical industry, because bubbles decrease the performance of electrodes. “Increasing our knowledge of the relationship between bubbles and electrochemistry will lead to design guidelines for high-performing electrochemical reactors, which are highly desirable in the chemical industry.”
Bubble formation
Fernandez Rivas describes the current knowledge of the formation of bubbles: “Bubbles typically form on cracks and crevices or other microscopic bumps in the electrode surface.” (see figure 1). An example of such a surface suitable for bubble formation is the edge of a glass. When the glass is filled with cola – or any other carbonated liquid – you can find, a nice ‘train’ of bubbles being formed from the dissolved carbon dioxide.
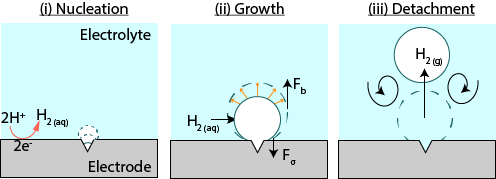
Figure 1 The formation of bubbles on the surface of an electrode
The bubbles on electrodes, however, can prevent the desired reaction from happening, which means the efficiency of the reaction is reduced. “A better understanding of the formation of bubbles can help us control their formation and thus increase the efficiency of reactions”, explains Fernandez Rivas.
Increasing efficiency
Several techniques to remove or reduce bubbles are available. One of which has been developed in Twente over the last decade makes use of microscopic engineered defects or pits on the surface of the electrode. The hydrophobic pit is where bubbles prefer to form (see figure 2). This technique makes it possible to form bubbles away from the electrode active surfaces.
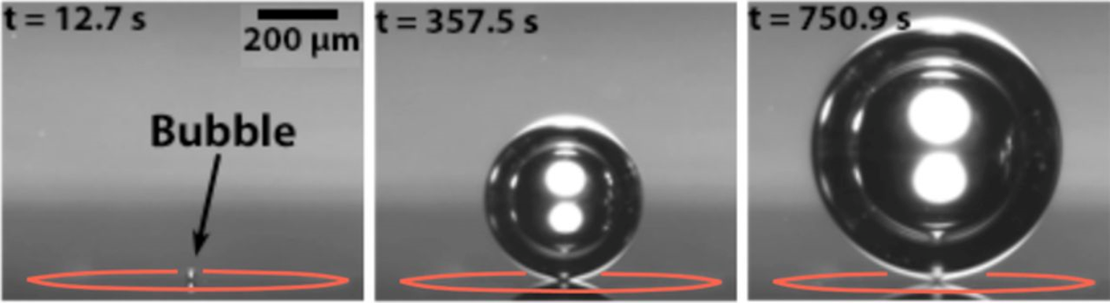
Figure 2 Bubble evolution at a hydrophobic pit
The formation away from the electrode active surfaces lets three things happen. First, each new bubble in a series takes longer to grow. Then a continuous gas production increases the local gas supersaturation which in turn actually increases the growth rate of bubbles. At last, the growth rate levels off to a steady growth rate. The formation of bubbles away from the electrodes may be used to minimize bubble-induced energy losses in electrochemical devices, like fuel cells.
Learn more about bubbles
The article, Influence of Bubbles on the Energy Conversion Efficiency of Electrochemical Reactors, was written by researchers from the Mesoscale Chemical Systems Group of the University of Twente and New York University. Their work was supported by the Netherlands Centre for Multiscale Catalytic Energy Conversion (MCEC), an NWO Gravitation programme funded by the Ministry of Education, Culture and Science of the government of the Netherlands, and by the Swiss National Science Foundation. The results were published today in the journal Joule.
More recent news
 Tue 16 Dec 2025Two UT projects receive Perspectief grant
Tue 16 Dec 2025Two UT projects receive Perspectief grant Tue 9 Dec 2025ERC Consolidator Grant for Dr Felix Gunkel
Tue 9 Dec 2025ERC Consolidator Grant for Dr Felix Gunkel Thu 4 Dec 2025The man who taught UT to grow
Thu 4 Dec 2025The man who taught UT to grow Fri 28 Nov 2025The Professor De Winter Award 2025 goes to Anneliene Jonker
Fri 28 Nov 2025The Professor De Winter Award 2025 goes to Anneliene Jonker Thu 27 Nov 2025NWO grant for sustainable cooling technology of the future
Thu 27 Nov 2025NWO grant for sustainable cooling technology of the future
