ELECTROCHEMICAL THIN FILMS AND INTERFACES

Group leader: Dr. Chris Baeumer
Challenge: store intermittent renewable energy
 We perform fundamental materials research in the light of today’s grand challenges: climate change, energy and resource efficiency. Renewable energy sources must replace current fossil fuel based technologies as soon as possible. This is difficult because of the intermittency of most renewable energy sources.
We perform fundamental materials research in the light of today’s grand challenges: climate change, energy and resource efficiency. Renewable energy sources must replace current fossil fuel based technologies as soon as possible. This is difficult because of the intermittency of most renewable energy sources.
Green hydrogen for energy storage
Therefore, energy transformation and storage options such as conversion to chemical fuel are necessary. The simplest and most attractive candidate for climate-neutral fuel and sustainable chemical synthesis is hydrogen produced by water splitting. Excess electricity from renewable energy can be used to split water into hydrogen and oxygen gas, and the energy stored in the hydrogen gas can be used later on or in a different process, effectively storing energy and coupling different sectors.
Materials Science: Designing efficient catalysts
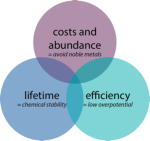 For this water-splitting reaction, catalyst materials are required, which reduce the amount of energy needed to generate a given amount of gas. These materials must be made of earth-abundant and safe materials, and must be efficient at catalysing the reaction to increase efficiency and stable under reaction conditions. We approach this fundamental research question through a special materials-by-design approach and through novel characterization tools.
For this water-splitting reaction, catalyst materials are required, which reduce the amount of energy needed to generate a given amount of gas. These materials must be made of earth-abundant and safe materials, and must be efficient at catalysing the reaction to increase efficiency and stable under reaction conditions. We approach this fundamental research question through a special materials-by-design approach and through novel characterization tools.
1. New catalyst materials and phenomena
We explore innovative catalyst materials and uncover the fundamental phenomena that govern their performance. This work is crucial for enhancing processes such as water electrolysis for green hydrogen production, where current catalysts often fall short in performance, cost, and sustainability for large‐scale applications.
2. Operando X-ray spectroscopy
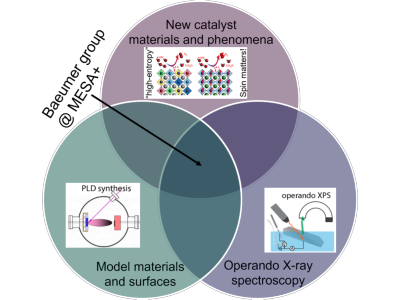 Recognizing that catalyst surfaces – the active site for all electrocatalytic reactions – evolve under reaction conditions, We utilize operando X-ray spectroscopy to observe the surface properties while the reaction is running. This approach provides dynamic insights that go beyond the limitations of “black box” optimization methods, which correlate as-prepared material properties with catalytic performance without capturing the active site evolution. Using operando spectroscopy, catalyst design can be based on activity, stability and selectivity trends with respect to physical and chemical properties of the true active surface.
Recognizing that catalyst surfaces – the active site for all electrocatalytic reactions – evolve under reaction conditions, We utilize operando X-ray spectroscopy to observe the surface properties while the reaction is running. This approach provides dynamic insights that go beyond the limitations of “black box” optimization methods, which correlate as-prepared material properties with catalytic performance without capturing the active site evolution. Using operando spectroscopy, catalyst design can be based on activity, stability and selectivity trends with respect to physical and chemical properties of the true active surface.
3. Atomically-defined model catalysts
We develop well-defined model catalysts, such as pulsed-laser deposited single crystalline thin films, which expose a single crystal facet with uniform morphology. This controlled surface offers a clear starting point for monitoring surface evolution during reactions – in contrast to traditional regular employing particulate-based catalysts embedded in a matrix of binder and support structures. My model catalysts also enable direct comparisons with ab-initio calculations, thereby integrating experimental observations with theoretical predictions further enhancing the predictive power of electrocatalyst research.
GROUP MISSION STATEMENT:
We are a group of “explorers” with diverse identities united by a shared mission. Our aim is to uncover fundamental structure-property-functionality relationships using innovative materials and methods. We push the boundaries of operando characterization techniques and develop new materials and phenomena for electrocatalysis, for example complex oxides and 2D materials. We facilitate collaboration between experimental and theoretical approaches, embedded in and contributing to the “H2 and green chemistry” communities at the University of Twente, in the Netherlands, and around the world.
At the core of our values is a commitment to fundamental understanding and to mission-oriented research towards a more sustainable world. We foster an environment of open communication, knowledge sharing, and mutual respect. We believe creative and impactful research result from diverse viewpoints, areas of expertise, personal backgrounds and an attitude of tackling hard challenges through team work. We encourage a critical mindset, embracing discussions about limitations and failures as opportunities for growth.
Our work revolves around materials and mechanisms in electrochemical reactions. We focus on model materials that allow understanding structure-property-functionality relations in electrocatalysis and use operando characterization to reveal properties under reaction conditions. Learning and research training are central for Bachelor, Master and PhD students and lifelong learning. Through interdisciplinary collaboration and open science practices, we aim to deliver expertise and contribute to the scientific community and society as a whole.
CURRENT RESEARCH PROJECTS:
Characterization during the reaction (“operando” science)
When we apply a voltage to catalysts in contact with water, multiple reactions occur simultaneously: While we begin to split water into oxygen and hydrogen, the surface of the catalysts changes the chemical composition, the structure and the electronic properties to adapt for reaction conditions. All of these properties determine if the material can be a good catalyst.
Some details are described in our recent publication . All of these properties determine if the material can be a good catalyst.
Therefore, we need to understand the true active state of the catalyst during the reaction to engineer more efficient and sustainable materials.
 We achieve this understanding using new characterization tools that can probe the catalyst surface during the water splitting reaction, including X-ray spectroscopy. The catalyst surface is especially important, because the changes here are crucial for reaction performance as that is where the reaction happen. These experiments are performed at the MESA+ Institute at the University of Twente and at international synchrotron facilities.
We achieve this understanding using new characterization tools that can probe the catalyst surface during the water splitting reaction, including X-ray spectroscopy. The catalyst surface is especially important, because the changes here are crucial for reaction performance as that is where the reaction happen. These experiments are performed at the MESA+ Institute at the University of Twente and at international synchrotron facilities.
The available techniques are summarised here.
The role of magnetic properties
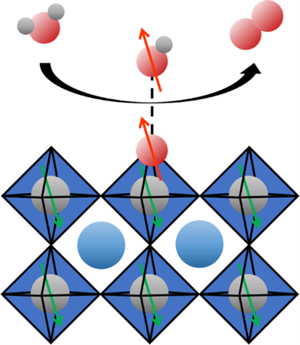
 State-of-the-art electrocatalyst still have an activity below the conceptual maximum. One reason can lie in the spin state of the reactants and products, for example in water splitting. The reactants are singlet, diamagnetic molecules, while the product oxygen molecule has a triplet, paramagnetic ground state with parallel spin alignment (↑O=O↑). It is thus proposed that using magnetic catalysts can enhance these reactions by enhancing the formation of the ground state as shown in the image below.
State-of-the-art electrocatalyst still have an activity below the conceptual maximum. One reason can lie in the spin state of the reactants and products, for example in water splitting. The reactants are singlet, diamagnetic molecules, while the product oxygen molecule has a triplet, paramagnetic ground state with parallel spin alignment (↑O=O↑). It is thus proposed that using magnetic catalysts can enhance these reactions by enhancing the formation of the ground state as shown in the image below.
 The focus of this work is to experimentally demonstrate the effects of intrinsic magnetic order in catalysts on their electrochemical activity and develop a deeper understanding of these effects. We try to find atomic structure-magnetic order-electrocatalytic activity relationships by investigating the efficiency of the oxygen evolution reaction during water splitting on epitaxial catalysts with a well-defined intrinsic magnetic order.
The focus of this work is to experimentally demonstrate the effects of intrinsic magnetic order in catalysts on their electrochemical activity and develop a deeper understanding of these effects. We try to find atomic structure-magnetic order-electrocatalytic activity relationships by investigating the efficiency of the oxygen evolution reaction during water splitting on epitaxial catalysts with a well-defined intrinsic magnetic order.
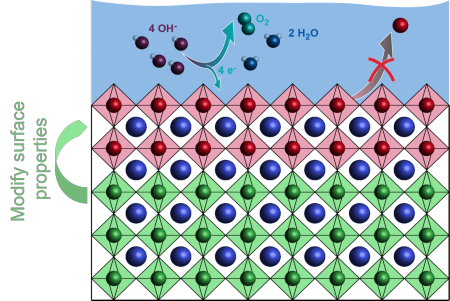
Bilayer electrocatalyst materials

 Many perovskite materials are active for the oxygen evolution reaction, but a fundamental challenge remains: the most active electrocatalysts are chemically unstable under reaction conditions. This limits the applicability of highly active electrocatalysts. Instead, high activity is often compromised by shorter lifetime. In our projects, we try to overcome this challenge by the use of multilayered perovskite materials. The focus lies on activation of ultrathin surface layers through the introduction of different subsurface layers. This enables new pathways to maximize the activity of chemically stable electrocatalysts.
Many perovskite materials are active for the oxygen evolution reaction, but a fundamental challenge remains: the most active electrocatalysts are chemically unstable under reaction conditions. This limits the applicability of highly active electrocatalysts. Instead, high activity is often compromised by shorter lifetime. In our projects, we try to overcome this challenge by the use of multilayered perovskite materials. The focus lies on activation of ultrathin surface layers through the introduction of different subsurface layers. This enables new pathways to maximize the activity of chemically stable electrocatalysts.
RESEARCH FACILITIES
We rely on advanced synthesis and characterisation tools, like pulsed laser deposition and the MESA+ operando HAXPES, and an electrochemical characterisation suite.
Explore our labs in this virtual tour.
FUNDING