The assignment below is an example of an assignment that can be chosen within the research theme chemistry of battery materials. We can shape your assignment as a bachelor or a master assignment, and also include your own ideas and interests. Assignments on closely related topics, e.g. energy-efficient direct recycling of battery electrodes, or development of a sustainable Na cathode phase are also possible.
More information? Contact André ten Elshof
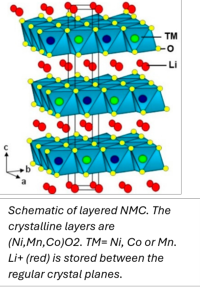
Assignment: Defect-free NMC cathodes for Li ion batteries
Li(Ni,Mn,Co)O2 (Li-NMC) is the most common cathode material for Li ion batteries in electric cars. In the current generation of cars, Ni-rich cathodes are used because they have the highest energy density of all Li-NMCs. A good example is NMC811, i.e. Ni0.8Mn0.1Co0.1O2. See image.
Unfortunately, the synthesis of NMC811 is hampered by substitutional defects that occur during synthesis: some Li+ ions swap positions with Ni2+ during the synthesis. The reason that this happens is due to thermodynamics (high entropy).
The substitutional Li+ ion is thereby immobilized at a “TM” position and lost for charge transport, while the Ni2+ ion ends up in the mobile Li+ layer where it blocks other Li+ ions and lowers the battery performance.
It would be much better if NMC811 without such substitutional defects can be made. In this assignment, you follow a two-step synthesis route: First, sodium NMC is synthesized through solid-state synthesis. The advantage of Na+ is that it is too large to fit into the regular Ni2+ position. The synthesis thus yields substitutional with defect-free crystal structure.
Secondly, an ion exchange process is carried out to replace all sodium ions with lithium ions. That process is done at such low temperature that the other ionic arrangements in the material are not affected.
The synthesized material's composition and structure will be analyzed using x-ray diffraction (XRD) for phase analysis and the determination of the interlayer spacing, electron microscopy (SEM) for morphology analysis, and X-ray fluorescence (XRF) to determine the atomic composition of the compounds. The concentration of substitutional defects present can be determined by Rietveld refinement. Selected electrodes will be tested electrochemically.
The goal of the assignment is to realize defect-free Li-NMC811 electrodes by the two step ion exchange method, and to explore if the resulting structure is stable upon charging and discharging (Li battery cycling). The research question to be answered is if the charging and discharging process also lead to substitutional defects, or that a stable NMC811 crystal structure is realized b y this method.
