The assignment below is an example of an assignment that can be chosen within the research theme chemistry of battery materials. We can shape your assignment as a bachelor or a master assignment, and also include your own ideas and interests. Assignments on closely related topics, e.g. energy-efficient direct recycling of battery electrodes, or development of a sustainable Na cathode phase are also possible.
More information? Contact André ten Elshof
Assignment: Chemical modification of Si nanoparticles for Li anodes
 Adding silicon to the carbon anodes of a lithium-ion battery is attractive because it increases the lithium capacity, and this positively influences the charge and energy density of the battery.
Adding silicon to the carbon anodes of a lithium-ion battery is attractive because it increases the lithium capacity, and this positively influences the charge and energy density of the battery.
Despite these possibilities there are some issues hampering the introduction of silicon anodes. One of the issues is that silicon experiences considerable expansion and contraction when alloying with lithium (Si + 4Li à Li4Si) and releasing Li, and this leads eventually to cracking of the electrode. Silicon nanoparticles are much better because they are small enough not to crack. But since nanoparticles are small they have a relatively huge surface area that is also the interface with their environment.
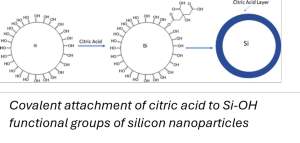
The reaction between the surface of silicon nanoparticles and the electrolyte during battery operation, can lead to electrolyte decomposition and the formation of a so-called solid electrolyte interlayer (SEI) at the surface. SEI layers can lead to enhanced degradation. A possible solution to this is surface modification of the silicon nanoparticles. See image.
In this assignment, silicon nanoparticles with either hydroxyl groups or siloxane bridges on their surface will be used for covalent attachment of surface functional groups. This involves a controlled sol-gel chemistry process. Surface modifications will be monitored using multiple techniques (including FTIR, thermogravimetric analysis and electrochemical testing).
