Microfluidic capturing of circulating tumor cells (CTC) from blood samples using Smart Sieves
Metastasis is the cause of death in 90% of cancer-related deaths. Screening of blood samples for the presence of tumor cells circulating in the blood stream –also known as CTCs- can give an indication of a patient's condition and prognosis. However, enumeration of CTCs is complicated since, typically, in a milliliter of blood, fewer than 10 CTCs are found, clouded by billions of red blood cells and millions of white blood cells [1].
Size-based capturing of CTCs relies on the fact that many CTCs are larger (10-30 mm) than red (≤ 8 mm) and white (~12 mm) blood cells. Conventional blood filters have pores of 5 mm diameter, but suffer from either low capturing yields of CTCs, much biofouling (due to lack of specificity) or low throughput. Furthermore, no technique currently allows screening more than few ml of blood due to both fouling of the membrane by blood cells and a low volumetric flow rate.
In this project, size-based capturing of CTC is combined with affinity-based capturing (see Figure 1). Microsieves are coated with an anti-biofouling polymer coating, on which antibodies are covalently immobilized. This should enhance the capturing efficiency and reduce clogging of the sieve by blood cells. Initial results show superior throughput as compared to conventional size-and-rigidity-based capturing. The goal is to enable screening of large samples (e.g. 5L in 24h). The assignment is to find the optimal process parameters corresponding to a situation where capturing efficiency of cancer cells is close to 100% and that of blood cells (fouling) is close to 0%. Optimization of the microsieve chip holder plays an important role in this project. Required: basic (bio)lab and fluorescence microscopy skills.
Skills that can be obtained, based on your preference: surface chemistry, cell culturing, image processing, microfluidics, 3D printing.
Keywords: (PDMS) microfluidics, antibodies, surface modification, circulating tumor cells, fluorescence microscopy, wearable diagnostics, Lab-on-a-Chip
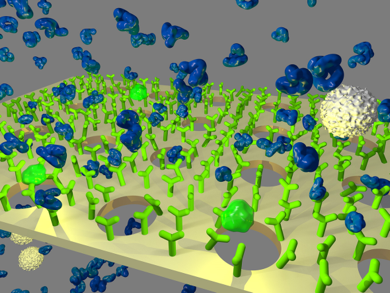
Figure 1: A Smart Sieve: a microsieve with covalently immobilized antibodies for selective capturing of analytes
References:
[1] de Wit, van Dalum, Lenferink, Tibbe, Hiltermann, Groen, van Rijn, Terstappen, Scientific Reports 5, 10 p., 12270
[2] Nguyen, Doorn van, Baggerman, Paulusse, Klerks, Zuilhof, Rijn van, Adv. Mat. Interfaces 2 (2015)
