The aim of the Cancer-ID program is to develop novel technology for monitoring Cancer therapy through revealing the extracellular vesicle IDentity (Cancer-ID). Novel technology platforms will be developed and validated for detection and detailed molecular characterization of cancer-associated EV. Isolation of EV from body fluids and staining protocols will be optimized, thereby enabling the optical, mechanical, biochemical characterization of EV, or sorted subpopulations thereof. This will enable the development of both macro and micro-fluidic devices to enumerate EVs, establish the cellular origin of single EV, and extract relevant information. These platforms will enable personalized medicine by efficiently monitoring disease development and therapeutic effectiveness, which will improve health care efficacy and reduce costs, thus ultimately improving the quality of life of patients.
Blood contains 106-1012 extracellular vesicles (EV) per mL originating from platelets, erythrocytes, and leukocytes. EV range in size from less than 100 nm in diameter to > 1 μm, with most EV having a diameter of < 200 nm.

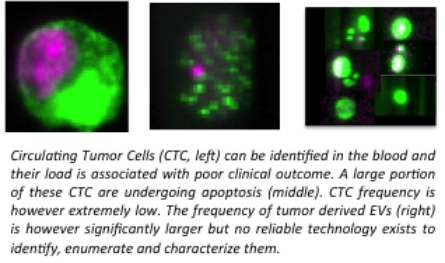
Isolation and establishing the cellular origin (identification) of single EV is extremely challenging because blood also contains platelets, which in size may overlap with larger EV (2-5 μm) and lipoprotein particles and protein aggregates which overlap in size and density with EV. Blood has a high density and viscosity and EV are heterogeneous in size and composition, which makes EV isolation even more difficult. The most commonly applied isolation protocols such as ultracentrifugation and density gradient centrifugation have serious draw-backs, resulting in massive and uncontrolled losses of EV subpopulations, poor recovery, contamination of for example protein aggregates, and loss of biological function. Thus, there is an urgent need for fast and reliable isolation / purification of EV from blood to gain access to EV as a novel source of biomarkers. In cancer, i.e. under pathological conditions, tumor-derived EV will enter the blood. Consequently, a fraction of EV in blood originates from cancer cells, and reliable identification of such EVs and the ability to examine their content can provide relevant information for the diagnosis, optimal therapy and monitoring of therapy.
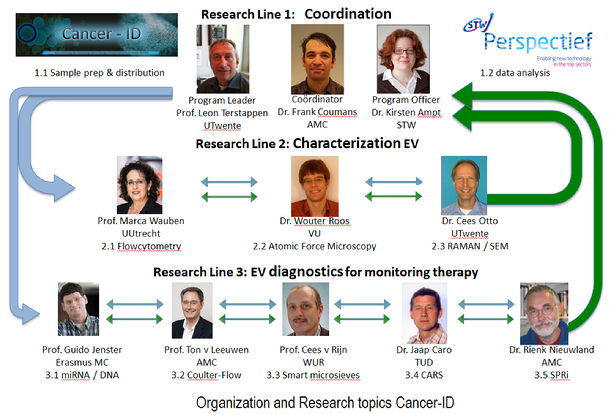
In this program 22 industrial partners combine their proprietary technologies with 11 fundamental and applied research groups to find a solution for reliable detection and characterization of EVs. The projects are interconnected, and combined knowhow will improve their capabilities and establish the next generation platform for EV diagnostics.