Organs-on-chips (OoC) platforms have revolutionized biomedical research by reducing animal testing and providing physiologically relevant models for studying organ function and drug responses in vitro. Mimicking the natural heartbeat rhythm in micro–Engineered Heart Tissues (microEHTs) is crucial for accurately simulating physiological conditions found in the human body and conducting high-throughput (HT) drug testing. However, achieving synchronized pacing of miniaturized cardiac strips formed in a 96-well plate presents a significant technical challenge, since the current system used for EHTs only stimulates the tissues one by one, which is not convenient for HT development. Moreover, cardiomyocytes’ (CMs) natural beating is spontaneous and varies in frequencies (Fig 1). But, for drug testing purposes, it is essential that they beat synchronously, which means they have the same relaxation/contraction cycle (Fig 2), to ensure consistency and reproducibility in experimental results.
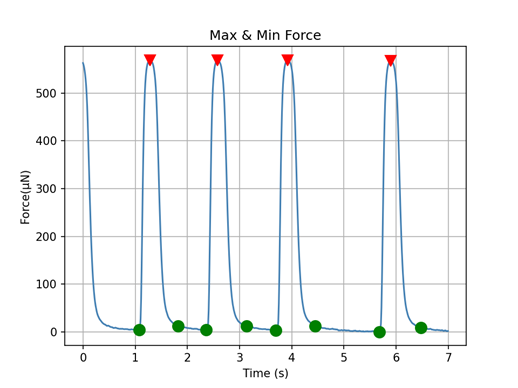
Figure 1. μ3D cardiac strips contraction force measurements.
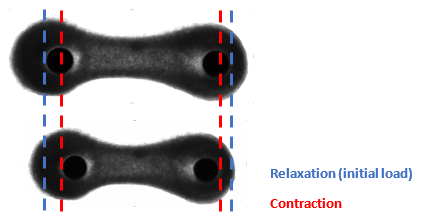
Figure 2. Relaxation mode refers to the initial load, which is the start of the measurement. Contraction is observed when cardiac tissues pull on the pillars.
This project aims to develop a parallel pacing system capable of stimulating multiple microEHTs simultaneously at the same frequency, thereby enhancing the efficiency and throughput of drug screening assays on microEHTs.
Project objectives:
- Develop a parallel and label-free pacing system capable of delivering synchronized electrical stimuli to miniaturized cardiac strips.
- Try different methods (e.g. photolithography, flexible electrodes 3D printing…).
- Integrate the pacing system with the cardiac microtiter plate to perform HT drug testing on microEHTs.
- The pacing system must be compatible with a 96-well plate format.
- μ3D cardiac tissues hanging on pillars must still be imageable from the bottom.
- Validate the pacing system compatibility using test experiments and computational simulations.
- Optimize the pacing parameters to ensure uniform and consistent pacing frequency and amplitude across all microEHTs.
- Evaluate the performance of the pacing system in maintaining physiological functionality and responsiveness of cardiac tissues.
This project is in close collaboration with River Biomedics.

