LiGalli, microfluidic chip.
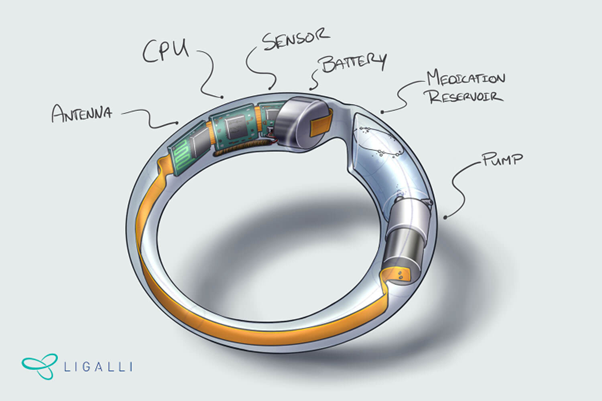
Historically, the participation of females in clinical trials has been underrepresented1. For example, in the US, in the period of 1977-1993 most woman of “childbearing potential” where banned from participating in clinical studies2. This underrepresentation causes large gaps in knowledge about gender specific medication, medical treatments, and toxicities of certain medications1. LiGalli is a company that is: on a mission to revolutionize women’s health care. LiGalli is trying to develop a smart vaginal ring for intra-vaginal sensing and is collaborating with BIOS on this subject.
The day-by-day research for the LiGalli project at the UTwente is run by two post-doctoral researchers, firstly, dr. Sevil Sahin is responsible for the sensing. Secondly, dr. Jasper Lozeman is responsible for the sampling and microfabrication. The project is further managed by two PI’s, namely professor Loes Segerink and professor Mathieu Odijk. This specific master assignment will be under the supervision of Jasper Lozeman and will focus on the microfluidics involved in the chip.
Microfluidics
The projects consist of several components, such as a sampling chamber and liquid storage. The microfluidics will need to connect all the different components together. The footprint of the microfluidics needs to be small enough to fit in the LiGalli ring. Eventually, a commercially viable device needs to be fabricated. This brings with it an additional challenge; the design should be relatively easy to be fabricated and mass produce. This means minimal use of cleanroom technology or any exotic materials.
For this assignment we look for a student that is interested in microfabrication and microfluidics. Since the project is in collaboration with several companies, the student should be willing to work on projects with a commercial application in mind.
Are you interested and do you want to know more? Please contact:
Dr. Jasper Lozeman j.j.a.lozeman@utwente.nl
Prof. dr. ir. Mathieu Odijkm.odijk@utwente.nl
1. Sosinsky, A. Z. et al. Enrollment of female participants in United States drug and device phase 1–3 clinical trials between 2016 and 2019. Contemp Clin Trials 115, 106718 (2022).
2. Merkatz, R. B. Inclusion of Women in Clinical Trials: A Historical Overview of Scientific Ethical and Legal Issues. Journal of Obstetric, Gynecologic & Neonatal Nursing 27, 78–84 (1998).
