CTCTrap aims to isolates and characterize all tumor cells circulating in blood to enable a real-time liquid biopsy for all cancer patients with metastatic disease regardless whether or not the disseminated disease has been clinically detected. The CTCTrap consortium consists of 4 Small & Medium Enterprises (SME) and 7 academic institutions and is funded through FP7 health.2012.1.2-1 #305341.
- Read more
Improving success rate of cancer therapy is strictly related to accurate selection of the patients that will benefit from certain therapies. However, despite the array of biomarkers some low-risk patients will die from distant metastasis whereas some high-risk patients will survive for decades. Moreover in the metastatic disease setting only a fraction of patients will respond to the selected therapy. Hence, the search for new clinical, biological or molecular tools that help the clinician in formulating, which patients will benefit from certain therapies is mandatory.
Metastasis is the major cause of death from cancer. In the past decade the traditional model of metastasis has been challenged by direct and indirect evidences, contrasting the view that tumor cells spreading to secondary sites is a late event in the tumorigenesis.
Peripheral blood represents an alternative minimally invasive source of spreading tumor cells. Circulating tumor cells (CTC) refer to cells that detach from a primary tumor or metastatic site that circulate in the peripheral blood and may settle down at secondary sites forming metastasis. In the past decade technology advances enabled the detection of these rare cancer cells shedding light on the disease' natural history and showing promise to serve as a liquid biopsy and used to tailor treatment for the individual patient. In current practice usually cancer tissue is taken at diagnosis and used to assess the presence of treatment targets. This however is suboptimal since tumor cells evolve due to genomic instability. Assessment of the genotype and phenotype of these CTC will provide insights into which treatments would be most beneficial for the individual patient.
Requirements for the use of CTC as a real time liquid biopsy are:
- CTC are indeed present in the sample volume
- CTC can be isolated from the blood
- CTC condition is sufficient to detect the presence of treatment targets
The CellSearch system selects CTC from 7.5 ml of blood based on the expression of both EpCAM and Cytokeratin 8, 18 or 19. With this system ~50% of patients with metastatic prostate and breast cancer have 5 or more CTC in 7.5 mL of blood before initiation of a new line of therapy. In the majority of these patients CTC are thus not available for therapeutic profiling. The use of less strict criteria to define CTC indeed will increase the number, but their condition is not sufficient for molecular characterization. Extrapolation of CTC frequency distribution in 7.5 ml of blood from patients with metastatic breast, colon and prostate cancer showed that probably all these patients had CTC in circulation, but the sample volume was not sufficient to detect them in all patients. (Coumans, et al ClinCanRes 2012). To arrive at a solution for this problem a significant larger blood volume if not all 5 liters will need to be analyzed.
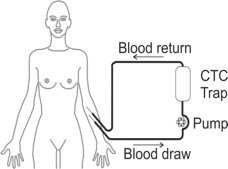
To eliminate this bottleneck the CTCTrap consortium develops Therapeutic Apheresis (TA), as a way to collect CTC from peripheral blood in cancer patients.
CTCTrap aims to:
- Develop safe and effective equipment for removal of CTC from 1-5 liters of peripheral blood.
- Phenotyping and genotyping of CTC, encompassing isolation and amplification of DNA&RNA from single CTC.
- Validate CTCapheresis in clinical use.
|
|
|
|
|
|
|
|
|
|
|
|










