Determination of protein activity by FRAP
BACKGROUND
Articular cartilage (kraakbeen) is a highly resilient tissue that covers the surfaces at the ends of long bones. Presence of cartilage ensures the pain-free and supple movements of our joints. Cartilage is mainly composed of one single cell type, the chondrocyte, that secretes the extra-cellular matrix (ECM) necessary for its load-bearing properties. Because of its avascular nature, and the embedding of chondrocytes in a dense ECM preventing cell migration, articular cartilage has low self-repair and regenerative capabilities. Therefore, if left untreated, cartilage injury results over time in complete erosion of the cartilage surface with arthroplasty (total joint replacement surgery) as the only treatment option.
All cells in the body, and thus also chondrocytes, respond to external signals are integrated into a specific cellular response. When cartilage tissue is damaged different signals are generated, causing inflammation and subsequent cartilage degeneration. These signals counteract and may overrule the intrinsic repair response of cartilage. To determine the optimal combination of bioactive factors that will promote cartilage formation after trauma, a better understanding of cell signaling in cartilage is needed.
The research activities of dr. Janine Post involve both the understanding of the complex signaling in healthy and damaged cartilage and modulating these signaling events by controlled release of kinase inhibitors in tissue engineering scaffolds.
In order to generate tissue engineering products for the repair of damaged cartilage, chondrocytes have to be isolated from a patient and expanded in culture. During this expansion in the lab the cells dedifferentiate to a more fibroblast-like cell type. Associated with this dedifferentiation are changes in gene expression and loss of the expression of collagen 2. However, the mechanism underlying the differentiation of these cells is not known. We hypothesize that the dedifferentiation of the chondrocytes is paired with a loss of kinase activity that interferes with the cell’s ability to react properly to the signals from its environment.
Among the signaling pathways important for cartilage formation and maintenance are the Wnt/beta-catenin signaling pathway and the BMP signaling pathway. Upon activation of the wnt signaling pathway beta-catenin accumulates in the cytoplasm and is subsequently transported into the nucleus. This results in activation of transcription of target genes that contain TCF-LEF transcription factor binding sites.
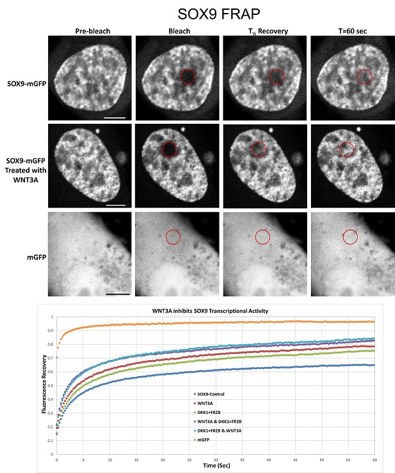
Activation of the wnt signaling pathway leads to downregulation of the transcription of SOX9, an important transcription factor in articular cartilage. Sox9 is necessary for the transcription of collagen 2, the most predominant type of collagen found in articular cartilage.
PROJECT
I have plasmids containing SOX9-GFP fusion constructs. After transfection into cells in culture, there will be abundant amounts of SOX9-GFP fusion proteins. Using this enables us to determine the subcellular localization of the proteins, the reaction of the protein localization to external signals such as WNT, BMP, but also catabolic agents as IL1β, TNFalpha, etc.
After determining the subcellular localization we will be able to determine the mobility of the proteins at their location using FRAP (fluorescent recovery after photobleaching).
Techniques that will be used in this project are:
- Cell culture (thawing, culturing, freezing cells)
- Transfection of DNA into cells
- Fluorescent microscopy to check green fluorescence
- Fluorescent laser scanning confocal microscopy for imaging of subcellular protein localization and FRAP
- Data analysis using modules in Image J
