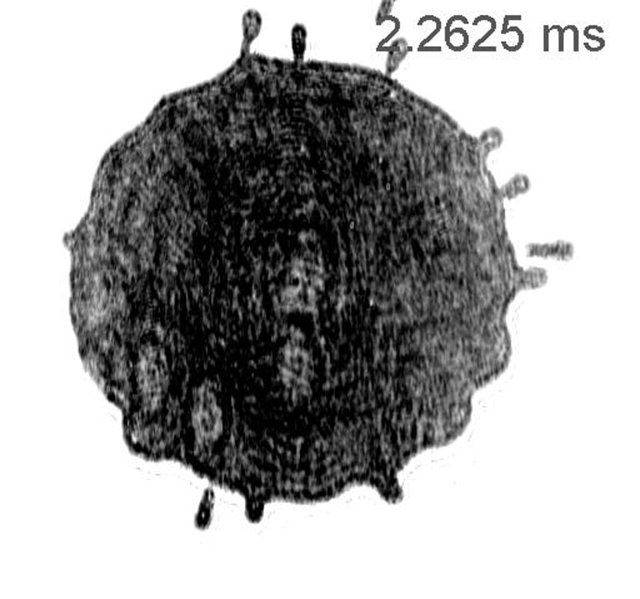
A droplet falling on a surface that is considerably supercooled, will freeze in a way that wasn’t ever observed before. Instead of the well-known growth of crystals, a colder surface results in moving circular ice fronts. These fronts move out the center to the edge of the freezing drop. Scientists of the University of Twente and the Max Planck Center for Complex Fluid Dynamics demonstrate this effect for the first time, and give an explanation for the physical mechanism involved, in the latest Proceedings of the National Academy of Sciences.
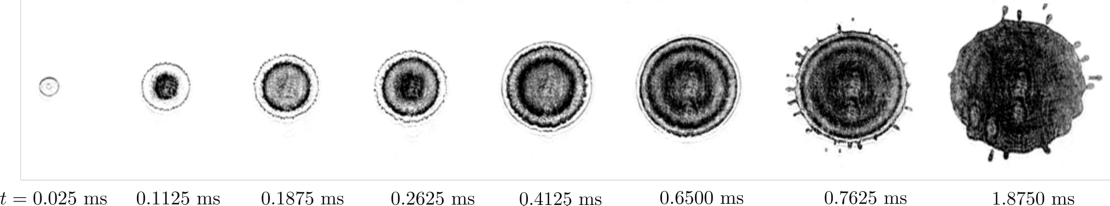
We know the risk of rain that is falling on a surface that is still frozen, making the road very slippery in a short time. It is an example of liquid droplets falling on a surface that has a temperature below the melting point – it is ‘supercooled’. The freezing of the droplet and crystallization remind us of the star-shaped dendritic structures often observed in snowflakes. Is the surface colder, however, the droplet not only freezes faster but the mechanism changes as well. At a surface that is cold enough, a remarkable phenomenon is observed: from the center of the droplet, ice fronts move towards the edge while the droplet is still spreading. This happens repeatedly, until the droplet is fully frozen.
Filming from below
The UT researchers observed this by filming the freezing of the droplet from the bottom, exactly at the surface. Laser light is reflected at the interface and filmed using a high speed camera. This is also called ‘total internal reflection’ (TIR), and is based on the same method that is used for taking fingerprints. In the experiments, the falling droplet is of hexadecane, which has a melting point of 18 degrees Celsius. The ‘waves’ were observed when the surface temperature was lowered to 11 degrees below this point.
Internal flow
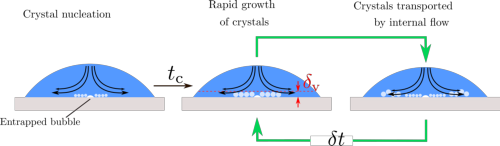
In their theoretical explanation in PNAS, the UT scientists show that the droplet is coldest at the point of impact, that is in the middle. Crystals form around this, but at the same time, the internal fluid flow pushes them to the boundaries. This process keeps repeating itself until the full droplet freezes. The study also reveals that the temperature of the surface changes the way the solified droplet attaches to the surface, thus changing the ease at which it can be ‘peeled off’.
The research not only gives fundamental insight in the process of freezing. It could help developing anti-icing surfaces, for airplanes for example. It can improve 3D printing techniques that make use of solidification of molten wax. And it can help further improving extreme ultraviolet lithography (EUV) for chip manufacturing. There, molten metallic droplets that solidify on mirrors could obstruct the whole process.
The research was in the Physics of Fluids group of UT’s MESA+ Institute and the Max Planck - University of Twente Center for Complex Fluid Dynamics, in which the university cooperates with a.o. the Max Planck Institute for Dynamics and Self-Organization in Göttingen, Germany.
The paper ‘Fast-freezing kinetics inside a droplet impacting on a cold surface’, by Pallav Kant, Robin Koldeweij, Kirtsten Harth, Michiel van Limbeek en Detlef Lohse, is published the latest Proceedings of the National Academy of Sciences of the USA (PNAS).